Angle of Contact
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Angle of Contact
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing the angle of contact, a very important concept in fluid mechanics. The angle of contact is defined as the angle between the tangent to the liquid surface at the point of contact and the solid surface inside the liquid. Can anyone explain why this concept is important?

It shows how a liquid behaves on different surfaces!

Exactly! It helps us understand whether a liquid will spread out on a surface or form droplets. Can anyone give an example of a situation where this matters?

Like how water beads up on a waxy surface but spreads on glass?

Great example! This relates back to the concept of wetting and contact angles. Let's remember a key point: a smaller angle means better wetting. Can anyone recall why this might be useful in, say, painting or coating?

If the liquid spreads better, it covers more area evenly!

Exactly! This spreading is crucial for applications like inkjet printing. To summarize, the angle of contact determines how a liquid interacts with a solid surface, impacting a variety of practical applications.
Interfacial Tensions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about the interfacial tensions at play when we look at the angle of contact. We have three key tensions to consider: liquid-air (Sla), solid-air (Ssa), and solid-liquid (Ssl). Does anyone remember the relationship between these tensions?

Sla cos(θ) + Ssl equals Ssa?

Correct! This relationship is fundamental in determining the angle of contact. It shows us how the attraction between materials affects the angle. If the liquid strongly attracts the solid, what happens to Ssl?

It decreases?

Yes! A smaller Ssl results in a smaller contact angle. To remember this, think of the mnemonic: ‘Tangles Mean Attraction’ - the more tangled or attracted the liquid is to the solid, the smaller the angle. Let’s provide a quick recap.

The contact angle is determined by interfacial tensions: liquid-air, solid-air, and solid-liquid tensions interact, and a smaller angle means better wetting.
Wetting and Practical Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore how the concept of the angle of contact applies practically. Can anyone describe a practical application of controlling wetting?

In waterproofing agents! They help create larger angles of contact so water beads on surfaces.

Exactly! Waterproofing agents increase the angle, making liquids less likely to wet the surface. This is important in outdoor gear and materials. What about cleaning agents?

They reduce the angle so liquids can spread and clean better!

Right! Soaps and detergents act as wetting agents. They lower the contact angle, which helps them penetrate surfaces more effectively. In short, understanding the angle of contact helps us in waterproofing, cleaning, and even in painting. Can anyone summarize why knowing this angle is beneficial?

It helps in designing products that better interact with liquids!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the angle of contact, denoted as θ, which varies among different liquid-solid interfaces. This angle affects how liquids behave when they contact solids, such as whether they spread out or form droplets. We also explore the relationships involving interfacial tensions at the contact points.
Detailed
Angle of Contact
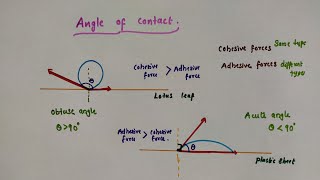
The angle of contact, or contact angle θ, is defined as the angle between the tangent to the liquid surface at the point of contact and the solid surface within the liquid. This angle varies with different liquid-solid combinations and plays a critical role in determining the wetting behavior of liquids on solid surfaces. If the angle of contact is acute (θ < 90°), the liquid wets the solid surface and spreads out, whereas if it is obtuse (θ > 90°), the liquid tends to form droplets.
Interfacial Tensions
The relationship of interfacial tensions at the three interfaces – liquid-air (Sla), solid-air (Ssa), and solid-liquid (Ssl) – is fundamental to explaining the concept of contact angle. At the line of contact, equilibrium must be maintained between these surface tensions, leading to the relation:
Sla cos(θ) + Ssl = Ssa.
This equation clarifies how different materials interact; strong liquid-solid attraction reduces the surface tension of the liquid-solids interface, resulting in a smaller angle of contact. Common examples include water exhibiting a high contact angle on a waxy leaf, forming droplets, versus low contact angles on glass, where it spreads. Additionally, wetting agents such as soaps and detergents lower the angle, promoting spreading, while waterproof agents can increase the contact angle, causing liquids to bead up.
Understanding the angle of contact is crucial in applications ranging from inkjet printing to the development of waterproofing materials, and it influences phenomena such as capillary action in narrow tubes.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Angle of Contact
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The angle between tangent to the liquid surface at the point of contact and solid surface inside the liquid is termed as angle of contact. It is denoted by θ.
Detailed Explanation
The angle of contact (or contact angle) is the angle formed at the interface of a liquid, solid, and gas. Specifically, it is measured between the tangent to the liquid's surface at the point where it meets the solid surface and the solid surface itself. This angle varies depending on the interaction of the liquid with the solid.
Examples & Analogies
Imagine placing a drop of water on a car's waxed surface. The water bead remains round and elevated, forming a larger angle with the surface of the car, representing a higher angle of contact. In contrast, if you drop water on a clean glass surface, it spreads out more and forms a smaller angle with the glass surface, indicating a lower angle of contact.
Effect of Angle of Contact on Spreading
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The value of θ determines whether a liquid will spread on the surface of a solid or it will form droplets on it.
Detailed Explanation
The angle of contact influences how a liquid behaves when it comes in contact with a solid surface. If the angle is acute (less than 90 degrees), the liquid tends to spread across the surface, effectively wetting it. Conversely, if the angle is obtuse (greater than 90 degrees), the liquid tends to bead up and form droplets without spreading, which is known as non-wetting.
Examples & Analogies
Consider a lotus leaf, which has a very high angle of contact with water. When raindrops land on the leaf, they bead up and slide off easily, demonstrating the concept of non-wetting, which is beneficial for the plant as it allows dirt to wash away. On a plastic plate, however, water spreads freely due to its lower angle of contact, illustrating how different materials interact variably with liquids.
Interfacial Tensions and Their Importance
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We consider the three interfacial tensions at all three interfaces, liquid-air, solid-air, and solid-liquid.
Detailed Explanation
At the line where the three phases meet (liquid, solid, and air), the balance of the interfacial tensions plays a critical role. The intermolecular forces at these interfaces create an equilibrium state. Understanding these forces helps in predicting whether a liquid will wet a solid surface or not and is crucial in various applications, such as coating technologies and in predicting how fluids behave in different environments.
Examples & Analogies
Think of how oil and water interact. Oil has a significantly different angle of contact with water because the interfacial tensions (solid-liquid and liquid-liquid) dictate that they don't mix, resulting in two distinct layers. This principle helps in processes like oil extraction, where understanding the interactions of liquids with different surfaces can lead to greater efficiency.
Obtuse vs. Acute Angles of Contact
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The angle of contact is an obtuse angle if S_sl > S_la and an acute angle if S_sl < S_la.
Detailed Explanation
The angle of contact provides insights into the degree of adhesion between the liquid and the solid surface. When the solid-liquid interfacial tension (S_sl) is stronger than the liquid-air interfacial tension (S_la), the angle θ is obtuse, causing the liquid to bead up. Conversely, when the liquid solid interaction is stronger, the angle θ is acute, and the liquid spreads out over the surface.
Examples & Analogies
For instance, if you apply a drop of water on a waxy surface, it beads up (obtuse angle) because wax repels water. In contrast, water on a clean glass surface (acute angle) spreads out due to strong adhesive forces between the glass and water molecules, illustrating how materials affect fluid behavior.
Key Concepts
-
Contact Angle: The angle at which a liquid interacts with a solid surface.
-
Interfacial Tensions: Forces at the interfaces among liquid, solid, and gas phases.
-
Wetting: How well a liquid spreads on a solid surface, dictated by the contact angle.
-
Wetting Agents: Substances that modify the contact angle to improve wetting.
Examples & Applications
Water on glass has a low contact angle, allowing it to spread (wetting), while water on wax has a high contact angle, causing beading.
Soap reduces the contact angle of water, allowing it to penetrate and clean effectively.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Contact angle dictates the spray, wet or bead, come what may.
Stories
Imagine pouring water on a glass table versus a wax surface. On glass, the water hugs and spreads out, but on wax, it just beads and runs away.
Memory Tools
Wishing A Wet Surface - W.A.W.S. reminds that a Wetting Agent lowers the angle!
Acronyms
WET - Wetting angle Explained by Tension refers to the understanding of wetting in relation to interfacial tensions.
Flash Cards
Glossary
- Angle of Contact
The angle formed between the tangent to the liquid surface at the point of contact and the solid surface within the liquid.
- Interfacial Tensions
The tensions present at the interface of different media, crucial for determining liquid behavior on solid surfaces.
- Wetting
The ability of a liquid to spread on a surface, influenced by the contact angle.
- Wetting Agents
Substances that reduce the angle of contact, increasing the ability of liquids to wet surfaces.
Reference links
Supplementary resources to enhance your learning experience.
