Drops and Bubbles
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Spheres in Drops and Bubbles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing why liquid drops and bubbles are spherical in shape. Can anyone think of a reason?

Is it because liquid tries to minimize its surface area?

Exactly! The sphere has the smallest surface area for a given volume, which makes it energetically favorable for the liquid.

So, does that mean if we ignored gravity completely, all liquids would form perfect spheres?

That's right! If gravity is negligible, the liquid will always take a spherical shape to minimize energy.

Let’s remember: 'Spheres for Surface Selection'—it's a memory aid that helps us recall this concept!

What role does surface tension play in this?

Surface tension is the key here. It creates pressure differences within the drops and bubbles, maintaining their structure.

To summarize, the spherical shape minimizes surface area and is energetically favorable when gravitational forces are minimal.
Pressure Differences Inside Drops and Bubbles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about the pressure inside drops and bubbles. Who can tell me how pressure is affected by the shape?

Is the pressure inside higher than outside?

Correct! For a spherical drop, we use the formula: $$(P_i - P_o) = \frac{2 \times S}{r}$$. Can anyone explain what each term means?

Here, P_i is the internal pressure, P_o is the external pressure, S is the surface tension, and r is the radius of the drop.

Well done! So, what happens in the case of a bubble with two surfaces?

The formula changes because we have to account for both surfaces, so it becomes $$(P_i - P_o) = \frac{4 \times S}{r}$$.

Exactly! This principle explains why blowing a bubble requires extra pressure. Remember, ‘Strong Bubbles Need Strong Pressure’!

In summary, the internal pressure of drops is affected by their radius and surface tension, maintaining their spherical structure.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Drops and bubbles are formed primarily due to surface tension, which causes liquids to minimize their surface area. This results in spherical shapes when external forces like gravity are negligible. The section also explains how pressure inside a drop or bubble is higher than the outside pressure due to surface tension effects.
Detailed
Drops and Bubbles
This section explores the phenomenon of drops and bubbles, emphasizing their spherical shape as a natural consequence of surface tension. When the effects of gravity are negligible, liquids tend to minimize their surface area, leading to spherical formations. Surface tension acts as an internal force that stabilizes the shape by creating a difference in pressure between the inside and outside of a liquid drop or bubble. For spherical drops, the pressure inside \(P_i\) is greater than the pressure outside \(P_o\) due to this surface tension, described mathematically as:
$$(P_i - P_o) = \frac{2 \times S}{r}$$
where S is the surface tension, and r is the radius of the drop. For bubbles, which have two liquid-gas interfaces, the excess pressure is calculated differently:
$$(P_i - P_o) = \frac{4 \times S}{r}$$
These equations signify the relationship between surface tension and pressure in liquid drops and bubbles, explaining why one needs to exert additional pressure to create or maintain soap bubbles. The understanding of these principles has implications not only in daily life but also in various scientific applications.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Spherical Shape of Drops and Bubbles
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
One consequence of surface tension is that free liquid drops and bubbles are spherical if effects of gravity can be neglected. You must have seen this especially clearly in small drops just formed in a high-speed spray or jet, and in soap bubbles blown by most of us in childhood. Why are drops and bubbles spherical? What keeps soap bubbles stable?
As we have been saying repeatedly, a liquid-air interface has energy, so for a given volume the surface with minimum energy is the one with the least area. The sphere has this property.
Detailed Explanation
Drops and bubbles take a spherical shape due to surface tension, which is the cohesive force that pulls liquid molecules together at the surface. When unaffected by gravity, a sphere has the smallest possible surface area for a given volume. This minimizes the surface energy of the liquid, making spherical shapes energetically favorable.
Examples & Analogies
Think about blowing up a balloon. As you inflate it, the air inside tries to equalize pressure on all sides, pushing out against the rubber. The balloon naturally tries to form a perfect sphere because that shape minimizes surface area relative to its volume—just like how drops of water behave when they fall.
Pressure Inside Drops and Bubbles
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
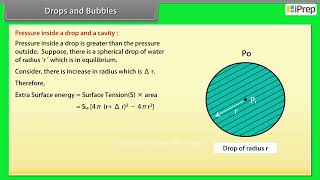
Another interesting consequence of surface tension is that the pressure inside a spherical drop is more than the pressure outside. Suppose a spherical drop of radius r is in equilibrium. If its radius increases by ∆r. The extra surface energy is
\[ [4\pi(r + \Delta r)^{2} - 4\pi r^{2}] S_{ha} = 8\pi r \Delta r S_{ha} \]
If the drop is in equilibrium this energy cost is balanced by the energy gain due to expansion under the pressure difference (Pi – Po) between the inside of the bubble and the outside. The work done is
$$ W = (P_1 - P_0) 4 \pi r^2 \Delta r $$ so that
\[(P_i - P_o) = \frac{2 S_{ln}}{r}\].
Detailed Explanation
For a spherical drop, the internal pressure (Pi) is greater than the external pressure (Po) due to the curvature created by surface tension. The equation shows how the force exerted by surface tension (Sla) balances the pressure difference across the surface of the drop. This relationship is key to understanding why small drops can hold together and maintain their shape despite the forces acting on them.
Examples & Analogies
Imagine blowing air into a balloon. As the balloon expands, the pressure inside increases because of the extra air volume that must be contained. Similarly, in tiny water droplets or soap bubbles, the pressure inside needs to be higher due to surface tension's effects, helping to keep them intact against external pressures.
Bubbles versus Drops
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A bubble differs from a drop and a cavity; in this it has two interfaces. Applying the above argument we have for a bubble \[(P_1 - P_0) = \left( \frac{4 S_{lh}}{r} \right)\]. This is probably why you have to blow hard, but not too hard, to form a soap bubble. A little extra air pressure is needed inside!
Detailed Explanation
Unlike a drop, which has one liquid-air interface, a bubble has two: one inside (where the air is) and one outside (where the liquid is). This means for a bubble, the internal pressure is even more elevated (by a factor related to the surface tension) than for a droplet. That’s why you need to apply a bit more force when blowing a bubble because you have to create enough air pressure to overcome this surface tension and form the double-surfaced structure.
Examples & Analogies
Remember when you were a child trying to blow big bubbles? You often had to find the right balance when blowing; too hard and the bubble would pop, but just the right amount and it would expand beautifully. This required understanding the forces at play—much like understanding how the pressure influences the stability of a bubble.
Capillary Rise and Surface Tension
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
One consequence of the pressure difference across a curved liquid-air interface is the well-known effect that water rises up in a narrow tube in spite of gravity.
Detailed Explanation
This phenomenon, known as capillary rise, occurs in narrow tubes due to the cohesive and adhesive forces acting on the liquid. Water is drawn into the tube against gravity because the adhesive force between the water molecules and the tube’s surface (which is greater than the cohesive forces within the liquid) causes the liquid to climb. The greater the surface tension, the higher the liquid will rise in the tube.
Examples & Analogies
Think of a straw in a glass of water. When you sip, the liquid rises up the straw, showing how liquid can counteract gravity due to these interactions. Similarly, if you dip a thin straw into water, you'll notice it draws water higher than what you'd see with a wider object; this highlights the importance of surface tension and adhesion in capillary action.
Key Concepts
-
Spherical Shape: Liquid drops and bubbles are spherical due to surface tension minimizing surface area.
-
Pressure Difference: The pressure inside a drop or bubble is higher than outside due to surface tension.
-
Excess Pressure: For drops, excess pressure is given by (2S/r) and for bubbles by (4S/r).
Examples & Applications
A raindrop falling forms a spherical shape due to surface tension, minimizing air resistance and energy.
Soap bubbles blown up by a child are examples of bubbles that illustrate the concept of excess pressure.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Drops are round, bright and fine, surface tension keeps them in line.
Stories
Imagine a tiny water droplet on a leaf, it wants to be as small as possible, creating a lovely, round shape.
Memory Tools
Remember Spheres for Surface Selection: S(urface area) is minimized by a Sphere!
Acronyms
PRES (Pressure Resting Inside Sphere)
The internal pressure of a drop or bubble.
Flash Cards
Glossary
- Surface Tension
The force acting at the surface of a liquid, causing it to behave as if its surface were covered with a stretched elastic membrane.
- Pressure
The force exerted per unit area on a surface.
- Spherical Drop
A drop of liquid that assumes a roughly spherical shape due to surface tension when gravity is negligible.
- Excess Pressure
The difference in pressure between the inside and outside of a bubble or drop.
Reference links
Supplementary resources to enhance your learning experience.
