Formation of Complex Compounds
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Complex Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, let's talk about complex compounds, especially those formed by transition metals. Can anyone tell me what a complex compound is?

A complex compound is made up of a metal ion attached to ligands, right?

Exactly! The metal ion acts as the central atom, surrounded by ligands, which can be either anions or neutral molecules. This unique configuration leads to some interesting properties.

Why are transition metals particularly good at forming these complexes?

Great question! Transition metals have smaller ionic sizes and higher charges, which allow them to form more stable bonds with ligands. Plus, they have available d orbitals that can overlap with ligand orbitals.

What are some examples of complex compounds?

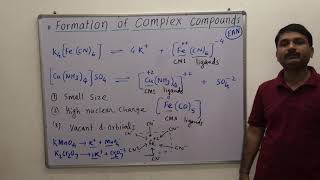
Some common ones include [Fe(CN)6]^{3-} and [Cu(NH3)4]^{2+}. They often exhibit distinctive colors and reactivities due to the metal-ligand interactions.

In summary, complex compounds are essential to understanding the chemistry of transition metals as they exhibit significant diversity in their structures and properties.
Properties and Applications of Complex Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we’ve introduced complex compounds, let's discuss their properties. What characteristics do you think contribute to their activity?

They have different colors depending on the ligands and the metal ion, right?

Exactly! The colors arise from electronic transitions within the d orbitals of the metal ions when they absorb specific wavelengths of light. This is also why coordination number and ligand type greatly influence the compound's properties.

What are some of the uses for these complex compounds?

Complex compounds find applications in various areas, including catalysis, medicine, and dye production. For instance, in catalysis, certain complexes can speed up reactions, making them more efficient.

As a summary, the ability of transition metals to form complex compounds not only contributes to their unique properties but also to their use in many practical applications across different fields.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Complex compounds involve metal ions bonded to various anions or neutral molecules, resulting in distinctly characterized species. The transition metals are particularly known for forming a vast array of such complex compounds due to their smaller ionic size, high charges, and availability of d orbitals for bonding.
Detailed
Formation of Complex Compounds
Transition metals are renowned for their ability to form complex compounds, which are defined as compounds consisting of a central metal ion surrounded by ligands, which can be anions or neutral molecules. This phenomenon is primarily attributed to the unique properties of transition metals, such as their smaller ionic sizes, greater positive charges, and the presence of d orbitals that can participate in bonding. Therefore, they can engage efficiently in complexation reactions.
The ability to form complexes grants transition metals a variety of characteristic properties, including varied coordination numbers and geometries, electronic configurations that allow for spectroscopic activity, and diverse reactivity. Common examples of complex compounds include [Fe(CN)6]^{3-} and [Cu(NH3)4]^{2+}. This capacity for forming complexes is important in both biological systems, for example, hemoglobin, and industrial applications, such as catalysts and batteries.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Complex Compounds
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Complex compounds are those in which the metal ions bind a number of anions or neutral molecules giving complex species with characteristic properties.
Detailed Explanation
Complex compounds consist of a central metal ion that is bonded to surrounding molecules or ions called ligands. These ligands can be negatively charged ions (anions) or neutral molecules. The unique interactions between the metal and ligands give rise to specific properties for each complex compound. For example, the coordination number, which indicates how many ligands can attach to the central metal, can vary based on the size and charge of the metal.
Examples & Analogies
Think of a complex compound like a chef (the metal ion) creating a signature dish (the complex compound) by combining various ingredients (the ligands). Just as each ingredient contributes to the final taste and presentation of the dish, each ligand influences the characteristics of the complex compound.
Examples of Complex Compounds
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A few examples are: [Fe(CN)6]2+, [Fe(CN)6]3-, [Cu(NH3)4]2+, and [PtCl4]2-. The chemistry of complex compounds is dealt with in detail in Unit 5.
Detailed Explanation
In the examples provided, each complex compound showcases a unique arrangement of a central metal ion with a specific number of ligands attached. For instance, in [Fe(CN)6]2+, iron is combined with six cyanide ligands; this results in a very stable structure. Similarly, [Cu(NH3)4]2+ has copper at the center, coordinated by four ammonia ligands. The manner in which these ligands arrange around the metal ion determines the stability, shape, and reactivity of the complex.
Examples & Analogies
Imagine assembling a team where each member has a specific role, like in a sports team. The central player (the metal ion) works with multiple teammates (the ligands), each bringing unique skills. Their arrangement and teamwork ultimately lead to how effectively the team can play—just as the arrangement of ligands around the metal determines the properties of the complex.
Factors Contributing to Formation of Complex Compounds
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The transition metals form a large number of complex compounds. This is due to the comparatively smaller sizes of the metal ions, their high ionic charges, and the availability of d orbitals for bond formation.
Detailed Explanation
Transition metals are particularly adept at forming complex compounds because they often possess unfilled d orbitals that can interact with ligands. The small size of transition metal ions allows for close interactions with ligands, while their high charge enables stronger attractions. Because of these factors, transition metals can create various complexes with different ligands, enhancing their chemical versatility.
Examples & Analogies
Consider a LEGO set where the transition metal is a specialized piece designed for unique connections. The smaller, high-charged metal pieces (like the transition metals) can connect with many different shapes (the ligands) to build various constructions (complex compounds). This explains why transition metals can create a diverse array of complex forms in chemistry.
Key Concepts
-
Complex Compounds: Compounds formed by a metal ion and ligands.
-
Ligands: Anions or neutral molecules that bond to metal ions.
Examples & Applications
Hexaamminecobalt(III) chloride: [Co(NH3)6]Cl3.
Tetraamminecopper(II) sulfate: Cu(NH3)4·H2O.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Metal plus ligand, what a blend, makes a complex, on that depend.
Stories
Once upon a time, in a chemistry village, a metal discovered it could dance with ligands, creating colorful complexes that shone in the laboratory.
Memory Tools
CML - Complex = Metal + Ligand.
Acronyms
COMPLEX - Central ion + One or more ligands = Metal bond, ligand engagement.
Flash Cards
Glossary
- Complex Compound
A compound consisting of a central metal ion bonded to one or more ligands.
- Ligand
An ion or molecule that binds to a central metal atom to form a complex.
Reference links
Supplementary resources to enhance your learning experience.
