Trends in the M²⁺/M Standard Electrode Potentials
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Standard Electrode Potentials
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re going to talk about standard electrode potentials related to transition metals. Can anyone explain what an electrode potential is?

Is it how likely a metal is to be oxidized or reduced?

Exactly! The standard electrode potential, E°, indicates the tendency of a metal to lose electrons. Generally, lower E° values indicate a stronger reducing agent. Now, let’s investigate the trend of E° across the 3d transition metals. What do we observe?

E° tends to become less negative as we move from titanium to zinc!

Correct! The trend reflects increasing ionization enthalpies. Remember this acronym: 'T-Z'. It stands for Titanium to Zinc, which marks that trend!

And what about copper? It has a positive E° value, right?

Yes! Copper’s positive E° explains why it doesn’t react with acids like many other metals. Any thoughts on why that is?

Maybe it's because the energy to turn Cu into Cu²⁺ isn't balanced by its stability as a hydrated ion?

Precisely! That’s an important takeaway regarding copper. To recap, standard electrode potentials illustrate trends across transition metals, highlighting unique behaviors such as those seen with copper.
Variability of Electrode Potentials
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Continuing from our last conversation, let’s delve deeper into the anomalies we see in electrode potentials, specifically looking at manganese and nickel. Can anyone tell me what trend we see with these metals?

Manganese has a more negative E° than expected, right?

Correct! Manganese's unique electron configuration contributes to its behavior. Why do you think this could affect its oxidizing or reducing potential?

Since Mn has a half-filled d subshell, it might be more stable in its oxidation states?

Exactly! And what about nickel? How does it compare?

Nickel also has a less negative E° than expected because of its high hydration enthalpy!

Spot on! The trends and anomalies in standard electrode potentials give us insight into the underlying stability and reactivity of these transition metals. Let’s summarize: E° trends not only indicate oxidation potentials but also give hints about chemical behaviors and electron configurations.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the trends in standard electrode potentials for the transition metals, focusing on the transformation from solid metal atoms to their ionized forms. The discussion includes the unique behavior of copper, the general trend in electrode potential values, and peculiarities observed for metals such as manganese and nickel.
Detailed
Trends in M2+/M Standard Electrode Potentials
This section analyzes the standard electrode potentials associated with the transition metals in their M2+/M forms, showcasing important trends throughout the series. The electrode potential, represented as E, informs us about a metal’s ability to exist in solution as ions.
Key Points Covered:
- Electrode Potentials Overview: The standard electrode potentials reveal how likely metals are to oxidize into their ionic forms. Lower (more negative) E° values indicate that a metal is more readily oxidized, making it a stronger reducing agent.
- Highlight on Copper: Copper showcases a positive E° value, which explains its reluctance to liberate H₂ from acids, emphasizing its unique reactivity compared to other transition metals.
- Trends Across Transition Metals: As we move across the series from titanium to zinc, a general trend of less negative E° values is observed. These trends are largely influenced by ionization enthalpies, which tend to increase across the series.
- Anomalies: Certain metals like manganese, nickel, and zinc show E° values that deviate from established trends, hinting at their unique stabilities and electronic configurations.
- Oxidation States and Stability: Discussion on why chromium and manganese present distinguished oxidation behaviors, notably how Cr acts as a reducing agent while Mn shows oxidizing properties.
Overall, understanding these potentials and trends is crucial for predicting the reactivity of transition metals and their compounds in various chemical environments.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Standard Electrode Potentials Overview
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
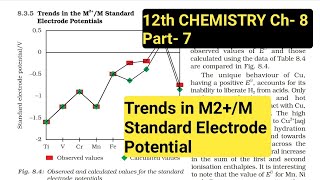
Table 4.4 contains the thermochemical parameters related to the transformation of the solid metal atoms to M ions in solution and their standard electrode potentials.
Detailed Explanation
This chunk discusses the standard electrode potentials (E° values) for various metals as they are converted from solid form to ions in solutions. It highlights the relationship between these potentials and the energy changes involved in the transformation of metals into ions, which is crucial for understanding their reactivity and behavior in chemical reactions.
Examples & Analogies
Imagine a metal being like a sealed can of soda. The standard electrode potential is like the pressure inside the can. When you open the can (transform the solid metal into ions), you release the pressure (energy), which helps us understand how likely the metal is to react with something else (like oxidizing or reducing agents).
Copper's Unique Behavior
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The unique behaviour of Cu, having a positive Eº, accounts for its inability to liberate H2 from acids.
Detailed Explanation
Copper exhibits a positive standard electrode potential (E°) which indicates that it does not readily lose electrons to react with acids, unlike many other metals. This is because the energy required to transform copper from solid to ion is not compensated by the energy gained through hydration of the ions formed, making it less reactive in acid solutions.
Examples & Analogies
Think of copper as a very polite person who refuses to engage in certain activities (like liberating hydrogen from acids). This is because, even though they might have something to gain in terms of coming into contact with the activity (transforming into Cu2+), the effort or energy required to do so isn’t worth it for them.
General Trends in Eº Values
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The general trend towards less negative Eº values across the series is related to the general increase in the sum of the first and second ionisation enthalpies.
Detailed Explanation
As you move across the series of transition metals, the standard electrode potentials tend to become less negative, indicating a trend where metals are becoming less likely to lose electrons. This trend is correlated with the increasing energy required to remove electrons due to higher ionization enthalpies.
Examples & Analogies
Imagine pushing a heavy ball up a hill. As you make the hill steeper (increasing ionization enthalpy), it becomes harder to push the ball upwards (lose an electron). So, the trend of the E° values reflects how hard or easy it is to push (lose electrons) as you go along the series.
Irregularities in Eº Values
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
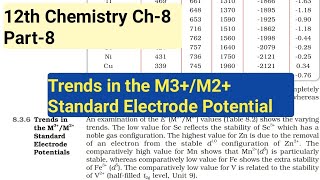
It is interesting to note that the value of Eº for Mn, Ni, and Zn are more negative than expected from the trend.
Detailed Explanation
While there is a general trend for E° values, Mn, Ni, and Zn show more negative E° values than one might predict based on their positions in the series. This suggests that these elements have unique interactions or stability that affect their reactivity.
Examples & Analogies
Imagine three friends planning to form a band. Although they all play similar instruments, some have natural talent for performing together that nobody expected. In this case, Mn, Ni, and Zn form stronger bonds or have special characteristics that make them react differently than the others.
Reducing and Oxidizing Strengths
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Why is Cr reducing and Mn oxidising when both have d configuration?
Detailed Explanation
Chromium (Cr) and manganese (Mn) can be seen in different electron configurations when they change oxidation states. Cr is considered a reducing agent due to its ability to easily give up electrons to achieve a more stable state. Conversely, Mn becomes more stable when it gains electrons, demonstrating its oxidizing strength.
Examples & Analogies
Think of Cr as a generous friend who readily shares their belongings (acts as a reducing agent) and Mn as a cautious friend who values their things (acts as an oxidizing agent). When pressure arises, these personalities reflect how they react differently in various situations.
Key Concepts
-
Standard Electrode Potential (E°): Indicates a metal's tendency to oxidize.
-
Trends Across Transition Metals: Generally less negative E° values from titanium to zinc.
-
Unique Copper Behavior: Exhibits a positive E° value, affecting its reactivity.
-
Anomalies: Certain metals display unexpected E° behavior due to specific electron configurations.
Examples & Applications
Copper (Cu) has a positive electrode potential of +0.34 V, making it a poor reducing agent.
Manganese (Mn) displays unexpectedly low E° values due to its d configuration.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Copper won't react, it stays pristine, / With its positive value, it's often seen.
Stories
Imagine a garden where copper flowers never wilt, signaling their power, yet never outside they yawn, reflecting how they don’t respond to standard acids.
Memory Tools
Use the acronym POTENTIAL: Positive means Oxygen (reducing agent), Negative means Total transformations occur.
Acronyms
TRUST
Titanium to Zinc - Relationships in Standard Electrode Trends.
Flash Cards
Glossary
- Electrode Potential (E°)
The measure of the tendency of a chemical species to be reduced; expressed in volts.
- Oxidizing Agent
A substance that gains electrons in a redox reaction and is reduced.
- Reducing Agent
A substance that loses electrons in a redox reaction and is oxidized.
- Halffilled d subshell
A d subshell that contains an exactly half set of electrons, often associated with increased stability.
Reference links
Supplementary resources to enhance your learning experience.
