Ionisation Enthalpies
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Trends in Ionisation Enthalpies
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to discuss ionisation enthalpies across the transition metals. Can anyone tell me what ionisation enthalpy actually is?

Isn't it the energy required to remove an electron from an atom?

Exactly! Now, as we move from left to right across the transition series, what trend do we see in ionisation enthalpies?

They increase, right?

But not as steeply as in the main group elements!

Correct! The increase is gradual and isn't as steep. This is due to several factors, such as increasing nuclear charge and electron shielding, among others. Can anyone explain how electron shielding affects ionisation energy?

The 3d electrons shield the 4s electrons more effectively, reducing the energy needed to remove them.

Great point! Remembering the concept of electron shielding is crucial. Think of it as d-electrons providing a cushion that softens the pull of the nucleus on the outermost 4s electrons. Let’s keep this in our minds for when we look at trends later.

To summarize, ionisation enthalpies generally increase across the transition series due to greater nuclear charge but the process is slowed down due to effective shielding. We will explore these concepts further in our next session.
Exceptional Ionisation Patterns
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

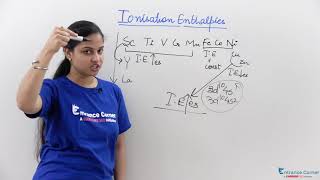
Now that we've established the trend in ionisation enthalpies, let’s discuss some exceptions. Can anyone recall a notable point where the trend deviates?

I remember you mentioned the transition from manganese to iron!

Exactly! When we remove one electron from manganese, it goes from a d5 configuration to a d6 configuration in iron. Why might that impact ionisation energy?

Because the d5 configuration is quite stable, right?

That’s correct! Stability in configurations like d5 influences how readily electrons are removed. So, the ionisation enthalpy for Mn2+ is lower than expected due to this stability. Always remember the balance between stability and energy while solving these problems.

Could you give us a mnemonic or something to remember this?

Sure! Think of 'Stable Mn'. It helps you remember manganese's stable d5 configuration and that its ionisation energy won't follow the expected pattern. Reflecting on this will assist in your understanding of the nuances involved.

In summary, while ionisation enthalpies tend to increase across the transition metals, specific configurations create exceptions, mainly due to stability in specific electron configurations.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Ionisation enthalpies of transition metals increase across the series from left to right due to increased nuclear charge and changes in electronic configurations. The section highlights that the trends in ionisation enthalpies are less steep compared to non-transition elements and explains factors contributing to this behaviour.
Detailed
Ionisation Enthalpies
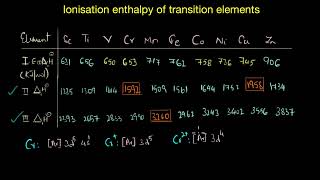
The ionisation enthalpies are an important aspect of the transition metals' chemistry. As we move from scandium to zinc in the 3d series of transition metals, we observe an increase in ionisation enthalpy due to the simultaneous filling of 3d orbitals coupled with an increase in nuclear charge. However, this increase is not as pronounced as in main group elements, and the trends exhibit unique characteristics.
Factors influencing these trends include:
- Electron Shielding: The 3d electrons provide a greater shielding effect on the 4s electrons than on themselves, thus influencing ionisation energies.
- Orbitals Energy Changes: The removal of electrons alters relative energies of 4s and 3d orbitals, leading to an irregular trend in ionisation enthalpies at specific points, such as the transition from Mn to Fe where d5 configurations are involved.
- Configuration Stability: The stability associated with half-filled and fully filled d subshells results in variations in effective nuclear charge perceived by the outermost electrons. This variation demonstrates why certain ions, like Mn2+ and Fe3+, show different ionisation characteristics despite being in the same d block.
In conclusion, the study of ionisation enthalpies across the transition metals provides insights into their chemical reactivity and stability. Understanding these trends is critical for predicting behaviour in reactions and compounds.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Ionisation Enthalpies
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
There is an increase in ionisation enthalpy along each series of the transition elements from left to right due to an increase in nuclear charge which accompanies the filling of the inner d orbitals.
Detailed Explanation
Ionisation enthalpy refers to the amount of energy required to remove an electron from an atom or ion in the gas phase. In the context of transition metals, as you move across a series from left to right, the nuclear charge increases because the number of protons in the nucleus also increases. This makes it harder to remove an electron, thereby increasing the ionisation enthalpy.
Examples & Analogies
Think of a magnet and a piece of metal. The stronger the magnet (higher nuclear charge), the harder it is to pull the metal away from it. This is similar to how higher nuclear charge makes it tougher to remove an electron, which reflects in higher ionisation enthalpy.
Trends in Ionisation Enthalpy
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The values of the first three ionisation enthalpies of the first series of transition elements show that the successive enthalpies of these elements do not increase as steeply as in the case of non-transition elements.
Detailed Explanation
In transition metals, while the first ionisation enthalpy generally increases, the increase in the second and third ionisation enthalpies is less pronounced. This means that removing more electrons from transition elements requires less additional energy compared to non-transition elements. This is due to the presence of d electrons which can sometimes shield the energy of the outermost electrons (the s electrons).
Examples & Analogies
Imagine trying to take a set of attached rings off a chain. The first ring might be tough to take off due to the tension in the chain. However, once you’ve removed the first few rings, the remaining rings are easier to remove because of the reduced tension.
Irregular Trends and Shielding Effect
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The irregular trend in the first ionisation enthalpy of the metals of the 3d series can be accounted for by considering the removal of one electron alters the relative energies of 4s and 3d orbitals.
Detailed Explanation
As electrons are removed from transition metals, the relative energies of the d and s orbitals can shift due to electron-electron interactions. For example, when an electron is removed from the 4s orbital, it may cause the 3d orbital electrons to become more stable due to reduced electron-electron repulsion, thereby impacting subsequent ionisation enthalpies.
Examples & Analogies
Consider a shaken soda can. When a can is shaken, the pressure builds up. The first time you open it, the pressure release is high. However, if you keep opening cans, they become easier to open because the fizzing action becomes less intense. Similarly, once one electron is removed, it can change the energy landscape for the remaining electrons.
Factors Affecting Ionisation Energies
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The interpretation of variation in ionisation enthalpy for an electronic configuration dn is as follows: the three terms responsible for the value of ionisation enthalpy are attraction of each electron towards nucleus, repulsion between the electrons and the exchange energy.
Detailed Explanation
The total ionisation energy can be influenced by three factors: 1) The attraction of electrons to the nucleus, 2) the repulsion between the electrons themselves (which can lower the energy required to remove one), and 3) exchange energy, which stabilizes electrons when they occupy degenerate orbitals with parallel spins (Hund's rule). When electrons are removed, their repulsion diminishes, which can also affect total ionisation energy.
Examples & Analogies
Think of a crowded room where everyone is pushing against each other (electron repulsion). If half of the people leave (removal of electrons), those remaining can move more freely, making it easier for others to exit the room too (lower ionisation energy required for subsequent removals).
Key Concepts
-
Ionisation Enthalpy: Energy required to remove an electron.
-
Nuclear Charge: Total positive charge from protons in the nucleus affecting ionisation.
-
Shielding Effect: Inner electrons reduce the nuclear charge's effect on outer electrons.
Examples & Applications
The first ionisation enthalpy for Scandium is lower than that for Zinc due to the filling of 3d electrons.
Manganese exhibits a lower ionisation energy due to its stable d5 configuration compared to the d6 configuration in Iron.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ionisation takes energy, To remove electrons, you see; Higher charge, the harder it seems, As we fill those electron dreams.
Stories
A student named Electra was curious about her transition metals. She discovered that as she walked across the periodic table, she needed more energy to pull each electron away due to the growing family of protons in the nucleus.
Memory Tools
Remember 'NSE' for Nuclear charge, Shielding, and Effective energy in ionisation enthalpy discussions.
Acronyms
Use 'ISH' - Ionisation, Shielding, and Hydrocarbons for remembering trends affecting ionisation energy.
Flash Cards
Glossary
- Ionisation Enthalpy
The energy required to remove an electron from an atom or ion.
- Nuclear Charge
The total charge of the nucleus, determined by the number of protons.
- Shielding Effect
The phenomenon where electrons in inner shells lessen the effective nuclear charge felt by outer shell electrons.
Reference links
Supplementary resources to enhance your learning experience.
