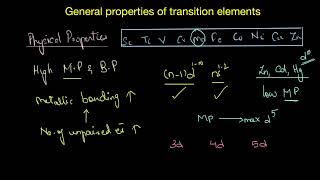
Physical Properties
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Transition Elements
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll discuss the primary physical properties of transition metals, like their high melting points and metallic characteristics. Can anyone tell me what makes metals conduct electricity well?

Is it because they have free-moving electrons?

Exactly! The mobility of electrons in metals facilitates conductivity. Transition metals have their d-electrons partially filled, enhancing this property. Remember the acronym 'MELT' for their Metallic properties: M for malleability, E for electrical conductivity, L for lustre, and T for tensile strength.

What about their melting points? I heard they are generally high?

Great observation! Their high melting and boiling points are due to strong metallic bonds generated from d-electron involvement. Now, who can explain the significance of having a high enthalpy of atomization?

Does it mean they have strong interatomic interactions?

That's correct! Strong bonds make them hard and dense. Let’s summarize: transition elements are generally hard, ductile, and have high melting points due to their strong metallic bonding.
Lattice Structures and Trends
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to lattice structures, transition metals can adopt different arrangements like hcp and ccp. Why do you think different structures affect their melting points?

Well, some structures allow atoms to be packed more closely, right? That would make bonds stronger.

Exactly! Structures like hcp allow for close packing, promoting stability and higher melting points. So, what trends do we see in melting points across the 3d, 4d, and 5d series?

They rise and then fall, right? Like a peak?

Yes! This is illustrated in the figures provided. The peak tends to occur around Mn for the 3d series. It shows how d-electrons contribute differently across the series. Remember that tendency: 'Peak around middle, drop at ends!'

Does that explain why some metals are less reactive?

Indeed! The stability of these properties indicates their reactivity. Let’s wrap up by noting the variety of lattice structures and how they influence physical properties!
Comparison of Transition Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s differentiate between the transition metals and the exceptions like Zn, Cd, and Hg. Why don't they follow the same property trends?

They have filled d orbitals, right?

That's correct! Completely filled d orbitals result in different bonding and conductivity behaviors. Using the mnemonic 'Zinc Sinks Hard', we can remember that Zinc, Cadmium, and Mercury show different properties.

How does that affect their applications?

Great question! For instance, Zn is less reactive compared to Cu, and thus is used differently in construction and industrial applications. Let's summarize key differences and properties regarding reactivity and stability.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Transition elements are characterized by their metallic properties, such as high tensile strength, ductility, and conductivity. Most exhibit high melting and boiling points due to the involvement of d electrons in metallic bonding. The arrangement of these elements in the periodic table impacts their lattice structures and melting points, demonstrating trends across different series.
Detailed
Physical Properties of Transition Elements
Transition elements are predominantly characterized by their metallic properties which include high tensile strength, ductility, malleability, high thermal and electrical conductivity, and metallic lustre. Notably, with the exceptions of Zn, Cd, Hg, and Mn, most transition elements maintain typical metallic lattice structures at standard temperatures, contributing to their physical robustness.
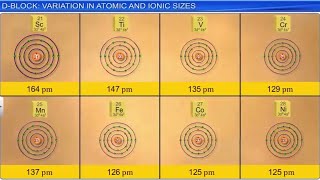
Lattice Structures: The transition metals can adopt different crystalline structures, including body-centered cubic (bcc), hexagonal close-packed (hcp), and cubic close-packed (ccp), depending on the element and its temperature. Figure queries for this section illustrate the diverse lattice formations and correlate these structures with the elements in the periodic table.
Melting Points and Hardness: Generally, transition metals demonstrate high melting and boiling points, with a notable peak around specific elements like Mn and Tc. This is due to the greater number of valence electrons participating in metallic bonding, providing increased strength in interatomic interactions. The melting point trends, illustrated in corresponding figures, decline regularly with a consistent increase in atomic number apart from some anomalous behaviors observed in specific metals.
In conclusion, transition metals exhibit a variety of metallic properties influenced by their unique electronic configurations and bonding capabilities, making them essential to industrial applications and modern technology.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Typical Metallic Properties
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Nearly all the transition elements display typical metallic properties such as high tensile strength, ductility, malleability, high thermal and electrical conductivity and metallic lustre. With the exceptions of Zn, Cd, Hg and Mn, they have one or more typical metallic structures at normal temperatures.
Detailed Explanation
Transition elements, which include metals like iron and copper, exhibit several common characteristics that define metals. This includes being able to be drawn into wires (ductility), hammered into sheets (malleability), and conducting heat and electricity efficiently. Most transition metals possess a shiny surface known as metallic lustre. However, there are a few exceptions like zinc (Zn), cadmium (Cd), mercury (Hg), and manganese (Mn) which may not exhibit all these qualities due to their unique electronic structure.
Examples & Analogies
Think of metals like copper and silver that are used in electrical wiring. They are not only able to conduct electricity well but also can be shaped into thin wires without breaking. This is similar to how a well-crafted piece of plastic can be molded; however, metals can do this even more effectively because of their ability to withstand stress without deforming or breaking.
Lattice Structures of Transition Metals
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Transition Metals have typical metallic structures such as hcp (hexagonal close packed), bcc (body-centred cubic), and ccp (cubic close packed). The specific lattice structures vary among the metals.
Detailed Explanation
The arrangement of atoms in a metal is crucial for understanding its properties. Transition metals can arrange themselves in different patterns known as lattice structures. For example, some metals arrange their atoms in a body-centered cubic formation (like iron) while others may form a face-centered cubic structure (such as copper). These patterns impact their strength, ductility, and how they conduct electricity and heat.
Examples & Analogies
Imagine how the layout of books on a shelf can affect how quickly you can find a specific book. If the books are organized neatly (like a structured lattice), you can locate what you need quickly. On the other hand, if the books are haphazard, it may take more time. Similarly, the arrangement of atoms in metals determines how effectively they behave under different conditions.
Physical Characteristics
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The transition metals (with the exception of Zn, Cd and Hg) are very hard and have low volatility. Their melting and boiling points are high.
Detailed Explanation
Most transition metals are known for their hardness, which makes them suitable for applications that require strong materials. They also have notably high melting and boiling points because of the strong metallic bonds present between the atoms. This is partly due to the involvement of d electrons in bonding, which adds to the overall strength of these interactions.
Examples & Analogies
Consider how durable tools like hammers and drills are typically made from strong metals. This durability is due to the metals' high melting points and hardness, which allow them to withstand high levels of stress and heat during use without deforming or melting.
Melting Points and Atomic Properties
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The high melting points of these metals are attributed to the involvement of greater number of electrons from (n-1)d in addition to the ns electrons in the interatomic metallic bonding.
Detailed Explanation
The melting points of transition metals can be understood through the concept of metallic bonding, where positively charged metal ions are surrounded by a 'sea' of delocalized electrons. The number of these delocalized electrons contributes to the strength of these bonds. Transition metals have more electrons available in their d orbitals, which strengthens these bonds compared to other types of metals, leading to higher melting and boiling points.
Examples & Analogies
Think of how an open fire depends on the amount and type of fuel available. Just like adding more fuel increases the heat of a fire, having more electrons involved in bonding makes metallic bonds stronger, resulting in higher melting points for transition metals.
Key Concepts
-
D-block Elements: Metals in Groups 3-12 characterized by their ability to form variable oxidation states.
-
High Melting and Boiling Points: Their strong metallic bonding results in these physical properties.
-
Lattice Structures: The arrangement affects stability and physical properties significantly.
-
Exceptions to Trends: Zn, Cd, and Hg possess filled d orbitals leading to different characteristics.
Examples & Applications
Example of a high-melting transition metal is tungsten, used in light bulb filaments.
Copper, a transition metal, demonstrates electrical conductivity due to its delocalized electrons.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Transition metals, strong and bold, with properties that never fold.
Stories
Imagine a castle of metal, with walls made from strong bonds of d-electrons, holding steadfast through heat and thunder.
Memory Tools
MELT for metallic properties: Malleability, Electrial conductivity, Lustre, Tensile strength.
Acronyms
For the lattice structure
'BCC
HCP
CCP – Beautiful Crystal Configurations in Metals'.
Flash Cards
Glossary
- Transition Elements
Elements found in groups 3-12 of the periodic table, characterized by the filling of d orbitals.
- Enthalpy of Atomization
The energy required to separate one mole of substance into its gaseous atoms.
- Lattice Structure
The arrangement of atoms in a crystalline solid, affecting its physical properties.
- Melting Point
The temperature at which a solid becomes a liquid.
- Metallic Bonding
The attraction between free-moving electrons and positively charged metal ions.
Reference links
Supplementary resources to enhance your learning experience.
