Trends in Stability of Higher Oxidation States
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Higher Oxidation States
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're diving into higher oxidation states of transition metals! Can anyone tell me why oxidation states are important in chemistry?

They show how many electrons an atom has lost or gained!

Exactly! The oxidation state reflects the electron transfer in chemical reactions. For transition metals, these states can vary greatly. For example, can anyone name a metal that exhibits a +7 oxidation state?

Maybe manganese? I remember it can go quite high.

Correct! Manganese can reach a +7 state, especially in compounds like Mn2O7. Let's remember the acronym 'M7O' for manganese's capacity for high oxidation states.

What makes those high oxidation states stable?

Great question! It's often the surrounding elements, like oxygen or fluorine, that stabilize these states. Let's look at this in our following session.
Stability of Halides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s focus on halides. Fluorine plays a significant role in stabilizing the highest oxidation states of metals. Can anyone think of an example?

I think titanium forms TiF4! Right?

Exactly! TiF4 is a tetrahalide. The stability due to fluorine's electronegativity helps stabilize these +4 oxidation states. Remember 'T4' for titanium's tetrahalide.

So, fluorine is basically helping metals stay in that high state?

Yes! And the same applies to chromium and vanadium as well. They tend to have stable compounds like CrF6 and VF5.

Are there any downsides to these compounds?

Certainly! While these compounds are stable, halides can be less so at lower oxidation states. Always keep an eye on stability trends!
Stability of Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving forward, let’s talk about oxides. Who can tell me how transition metals display their highest oxidation states in oxides?

Uh, do they form things like MnO2 or maybe CrO3?

Yes! Manganese can also exist in Mn2O7, which exceeds those simple states. This shows how important oxides are!

Why do we think oxygen is better than fluorine in stabilizing these high states?

Great thought! Oxygen can form multiple bonds with metals, enhancing stability. We can summarize this with 'O2=stability'.

So, higher oxidation states mostly lead to more complex bonding?

Correct! It’s the complexity of bonding that enhances stability. Always remember: more connections mean more stability!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details how the highest oxidation states for 3d transition metals are represented in specific compounds, emphasizing the significant role of fluorine and oxygen in stabilizing these states. It addresses the properties of halides and oxides formed by transition metals and highlights variations in oxidation states across the series.
Detailed
Trends in Stability of Higher Oxidation States
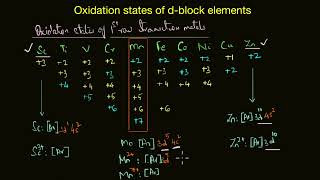
This section examines the stability of higher oxidation states among transition metals, particularly within the context of their halides and oxides. Transition metals can exhibit multiple oxidation states, which is a key characteristic of these elements.
- Halides: The ability of certain transition metals to achieve high oxidation numbers is significant. For example, titanium forms tetrahalides such as TiX4, while vanadium and chromium achieve high oxidation states through their respective halides. Notably, the presence of fluorine stabilizes these high states due to its high lattice energy and bond enthalpy, making compounds like VF5 and CrF6 notably stable.
- Oxides: Transition metals also showcase a range of oxidation states in their oxides. For example, manganese in Mn2O7 reaches +7 oxidation, which is higher than what is typically found in simple halides. The capability of oxygen to stabilize high oxidation states surpasses that of fluorine, particularly in covalent compounds.
In summary, the transition metals demonstrate remarkable variability in oxidation states, which is influenced significantly by the nature of the compounds they form, particularly in the presence of strong oxidizing agents like fluorine and oxygen. This chapter emphasizes the stability trends of oxidation states and their implications in the chemistry of transition metals.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Higher Oxidation States
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Table 4.5 shows the stable halides of the 3d series of transition metals. The highest oxidation numbers are achieved in TiX4 (tetrahalides), VF5 and CrF6. The +7 state for Mn is not represented in simple halides but MnO3F is known, and beyond Mn no metal has a trihalide except FeX3 and CoF3.
Detailed Explanation
This chunk introduces the concept of higher oxidation states of transition metals, particularly focusing on the stability of higher oxidation numbers among various element halides. Notably, titanium allows for tetrahalides, vanadium for pentahalides, and chromium for hexahalides. The chunk highlights that manganese can achieve a +7 oxidation state, which is not found in simple halides like the others.
Examples & Analogies
Think of oxidation states like levels in a video game. Each level (or oxidation state) represents a different challenge and capability. Some metals can reach higher levels because they possess more energy and capabilities (like electrons) to stabilize those higher states, while others are restricted to lower levels.
Factors Affecting Stability
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ability of fluorine to stabilise the highest oxidation state is due to either higher lattice energy as in the case of CoF3, or higher bond enthalpy terms for the higher covalent compounds, e.g., VF5 and CrF6.
Detailed Explanation
This section focuses on why fluorine can help transition metals maintain their highest oxidation states. It mentions two main factors: lattice energy, which applies to ionic compounds like CoF3, and bond enthalpy for covalent compounds like VF5 and CrF6. Lattice energy refers to the energy released when ionic compounds form a stable crystal lattice, while bond enthalpy is the energy required to break a bond between atoms in a molecule.
Examples & Analogies
Imagine building a strong Lego structure. A low-energy structure (like lattice energy) holds the blocks tightly together, making it hard to break apart. Similarly, higher bond strength (like bond enthalpy) keeps the connections between the blocks secure. Thus, the stronger the energy binding the atoms, the more stable the entire structure (oxidation state) is.
Hydrolysis and Oxohalides Formation
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
+5 Although V is represented only by VF5, the other halides, however, undergo hydrolysis to give oxohalides, VOX3. Another feature of fluorides is their instability in the low oxidation states e.g., VX2 (X = CI, Br or I).
Detailed Explanation
The chunk discusses that while vanadium's highest oxidation state is shown in the compound VF5, other halides will react with water (hydrolyze) to produce oxohalides like VOX3. Additionally, it highlights the instability of fluorides formed in lower oxidation states, like VX2, when compared to their higher oxidation states. This suggests that having fewer electrons or a lower oxidation state makes these compounds less stable.
Examples & Analogies
Consider how a lightweight balloon can easily drift away (unstable) compared to a heavy rock on the ground (stable). The balloon represents the low oxidation states that quickly change or break down, while the rock represents the higher oxidation states that are more stable and less likely to change.
Copper Halides and Stability
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation.
Detailed Explanation
This part elucidates the instability of copper (I) compounds when they dissolve in water, leading to a process called disproportionation, where copper (I) is split into copper metal and another higher oxidation state of copper. This reaction illustrates the tendency of some metals to become less stable in lower oxidation states when in solution.
Examples & Analogies
You can think of it as a bridge made of weak materials. When pressure from the weight above increases too much, the bridge collapses and falls apart, similar to how unstable copper (I) compounds fall apart in solution to form more stable forms of copper.
Oxygen's Role in Stabilizing Oxidation States
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ability of oxygen to stabilise the highest oxidation state is demonstrated in the oxides. The highest oxidation number in the oxides (Table 4.6) coincides with the group number and is attained in Sc2O3 to Mn2O7.
Detailed Explanation
This section explains how elements can stabilize their highest oxidation states when forming oxides. The maximum oxidation state corresponds to the group number of the element, illustrating that elements from scandium to manganese can reach their top oxidation states in their oxides. This is critical as it shows how oxygen can bond with metals in a way that stabilizes higher oxidation states than other elements.
Examples & Analogies
Like a tree that supports its branches to grow higher, oxygen acts as a supporting material that enables these metals to reach and hold more electrons, stabilizing their higher oxidation states. The presence of oxygen thus allows these metals to climb higher in their oxidation state 'tree'.
Exploring Oxides and Oxocations
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The highest oxidation number in the oxides coincides with the group number and is attained in Sc2O3 to Mn2O7. Beyond Group 7, no higher oxides of Fe above Fe2O3 are known, although ferrates (VI)(FeO4)2–, are formed in alkaline media but they readily decompose to Fe2O3 and O2.
Detailed Explanation
This chunk emphasizes the correlation between the highest oxidation states of transition metals in their oxides and their respective group numbers in the periodic table. It notes that while manganese can reach a high +7 oxidation state in oxides, iron is limited to +3 in most scenarios, reflecting its inability to stabilize higher oxidation states in oxidized forms. It also points out the decomposition of complex ferrates back to stable states.
Examples & Analogies
Consider it like a mountain range where higher peaks represent high oxidation states. Some mountains (like iron) have high limits they can’t surpass (maximum +3) due to their geological structure, while others (like manganese) allow for much greater heights (up to +7), demonstrating the correlation between the environment (chemical structure) and the peak heights (oxidation states).
Key Concepts
-
Fluorine stabilizes high oxidation states: High lattice energy and bond enthalpy.
-
Oxygen's superior stabilizing ability: Multiple bonding enhances stability of oxides.
-
Variety in oxidation states: Transition metals show diverse oxidation states.
Examples & Applications
Titanium forms TiF4 as a stable tetrahalide.
Manganese demonstrates +7 oxidation state in Mn2O7.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Titanium goes high, with fluorine nearby, TiF4 – that's the reason, for a stable season.
Stories
Picture a strong bond where titanium meets fluorine, creating a mighty TiF4 mountain top, secure and high, nothing can drop.
Memory Tools
For titanium in tetrahalides, think 'T4' – strong and alive!
Acronyms
Use 'H.O.F' to remember Fluorine's stabilizing power
High Oxidation stability Factor.
Flash Cards
Glossary
- Oxidation State
The total number of electrons that an atom can lose, gain, or share in a compound.
- Tetrahalides
Compounds formed from a metal and four halogen atoms.
- Halides
Compounds that consist of a halogen and another element.
- Oxides
Compounds formed from oxygen and another element.
- Stability
The tendency of a compound to maintain its oxidation state under various conditions.
Reference links
Supplementary resources to enhance your learning experience.
