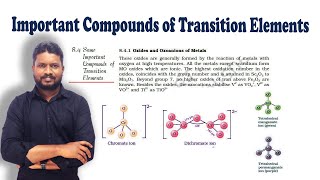
Oxides and Oxoanions of Metals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Formation of Metal Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we’re going to explore how metal oxides are formed. Can anyone tell me how metals typically react to form oxides?

I think metals react with oxygen at high temperatures to form oxides.

Exactly! These reactions usually produce ionic compounds, represented as MO, where M is the metal. Can anyone give an example of this?

Maybe iron oxide since iron reacts with oxygen to form rust?

Good example! Moreover, the highest oxidation state of these metals correlates with their group number. For instance, manganese can reach a +7 oxidation state in MnO4. What does this imply about its reactivity?

That means manganese can act as a powerful oxidizing agent!

Exactly! Let’s remember that as we move forward. Oxides can show varying degrees of acidity and basicity depending on the metal involved.
Key Oxoanions: Dichromate and Permanganate
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about specific oxoanions like potassium dichromate. What is its significance in chemistry?

I know that potassium dichromate is used in the leather industry as an oxidizing agent.

Correct! Can anyone describe how potassium dichromate is formed?

It’s produced by fusing chromite ore with sodium carbonate in the presence of oxygen.

Well done! And what about potassium permanganate? How is it synthesized?

It’s made by heating MnO with potassium hydroxide and an oxidizing agent.

Great job! The dark purple crystals of potassium permanganate can oxidize various compounds. Can someone give an example?

I’ve seen it oxidize iodide ions to iodine!

Exactly! Remember this reaction when we discuss redox processes next week.
Properties of Oxides and Oxoanions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive into the properties of metal oxides. How does the ionic character change with increasing oxidation states?

As the oxidation state increases, the ionic character generally decreases.

Right! Higher oxidation states often lead to more covalent characteristics. Can anyone describe the acidity of certain oxides?

Some metal oxides become acidic, such as chromium oxide.

Exactly! For instance, CrO3 gives H2CrO4, demonstrating its acidic property. What about amphoteric oxides?

I think vanadium oxides can act amphoterically, behaving as both acids and bases!

Good memory! V2O5 dissolves in both acids and bases to form relevant oxoanions. Adapting to conditions is essential for understanding these compounds.
Redox Reactions Involving Oxoanions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand oxoanions, how do they participate in redox reactions?

They can either be reduced or oxidized depending on the reaction conditions.

Exactly correct! For example, potassium permanganate can be reduced from MnO4- to MnO2 in neutral solutions. Why is the oxidation state important here?

It helps to track the loss or gain of electrons during the reactions!

Absolutely! And as we progress, keep in mind that balancing these half-reactions is key for stoichiometry in redox reactions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section captures key aspects of how metal oxides are formed, particularly through reactions with oxygen at high temperatures. It addresses the behavior of various metals in terms of their oxidation states and introduces specific examples, such as potassium dichromate and potassium permanganate, emphasizing their significance in industrial applications and chemistry.
Detailed
Oxides and Oxoanions of Metals
In this section, we explore the formation and characteristics of metal oxides formed by reactions with oxygen compounds, primarily at elevated temperatures. Most metals, except for scandium, form ionic oxides represented as MO. The highest oxidation state of metals in the oxides generally matches their group number and displays significant variations in chemical behavior. A key focus is manganese, where MnO2 exhibits covalent properties despite being a metal oxide. The acidity and basicity of these oxides vary, with some displaying amphoteric characteristics.
We also discuss key examples like potassium dichromate, which is utilized in diverse chemical processes, and potassium permanganate, emphasizing their properties and roles as strong oxidizing agents. The section highlights the transition from metal oxides to oxoanions, illustrating the conversion of chromate to dichromate under varying pH conditions. Notably, we note the oxidative capabilities of these chemical species, their formation, and pertinent reactions which play significant roles in both laboratory and industrial settings.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Formation of Oxides
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These oxides are generally formed by the reaction of metals with compounds of oxygen at high temperatures. All the metals except scandium form MO oxides which are ionic.
Detailed Explanation
Oxides are chemical compounds made up of oxygen and another element, typically a metal. When metals react with oxygen sources (such as oxides or molecular oxygen), they can form metal oxides (MO). The reaction often requires high temperatures to take place effectively. Most metals, with the exception of scandium, will form these ionic compounds.
Examples & Analogies
Think of oxides like a metal wearing a coat made of oxygen during a reaction. Just as we may need heat to make certain materials melt together, metals need high temperatures to successfully combine with oxygen to form their 'coat' (oxide).
Oxidation States of Metals in Oxides
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The highest oxidation number in the oxides coincides with the group number and is attained in Sc2O3 and MnO2. Beyond group 7, no higher oxides of iron above Fe2O3 are known.
Detailed Explanation
The oxidation state of a metal in an oxide typically corresponds to the group it belongs to in the periodic table. For example, a metal in group 3 may have an oxidation state of +3 when forming oxides such as Sc2O3. However, as we move beyond group 7, the ability of metals like iron to form higher oxides diminishes, with Fe2O3 being the highest common oxide observed for iron.
Examples & Analogies
Imagine a scoreboard showing the highest possible score a player can reach (the oxidation number). Just as a player in a basketball game can only score a certain number of points depending on their position (their group), metals can only reach a specific oxidation number based on their group in the periodic table.
Acidic Character of Higher Oxides
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In these higher oxides, the acidic character is predominant. Thus, Mn2O7 gives H-MnO4 and CrO3 gives H2CrO4.
Detailed Explanation
Higher oxides of certain metals exhibit acidic properties. For instance, when manganese in the oxidation state +7 forms Mn2O7, it results in an acidic solution when reacted with water to produce H-MnO4. Similarly, chromium in the +6 state reacts to form an acid, H2CrO4, indicating that higher oxidation states contribute to stronger acidic characteristics in these metal oxides.
Examples & Analogies
Think of these higher oxides as powerful ingredients in a recipe for a sour dish. Just like some ingredients can make food taste sour and acidic, the higher oxidation states of metals in oxides can create acids when combined with water, showing their 'sour' (acidic) nature.
Amphoteric and Basic Character of Oxides
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
V2O5 is, however, amphoteric though mainly acidic and it gives VO3– as well as VO2+ salts. In vanadium, there is gradual change from the basic V2O3 to less basic V2O5 and to amphoteric V2O4.
Detailed Explanation
Vanadium's oxides behave differently based on their oxidation states. V2O5 is usually acidic but can also act amphoteric, meaning it can react with both acids and bases. The classification of oxides varies along the series, where V2O3 exhibits basic behavior, V2O5 is less basic, and V2O4 shows amphoteric properties. This indicates a transition in behavior as the oxidation state of vanadium changes.
Examples & Analogies
Imagine how some people can be really flexible in a discussion. They can agree with one side (acting like a base with acids) while being firm on another point (acting like an acid with bases). Similarly, some metal oxides can switch roles, behaving differently in chemical reactions depending on the circumstances.
Preparation and Use of Potassium Dichromate
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Potassium dichromate is a very important chemical used in leather industry and as an oxidant for preparation of many azo compounds.
Detailed Explanation
Potassium dichromate (K2Cr2O7) is a crucial chemical compound used across various industries, notably in leather tanning and organic chemical synthesis. It acts as a strong oxidizing agent, facilitating reactions that form azo compounds, which are used for dyes.
Examples & Analogies
Think of potassium dichromate as a very busy helper in a factory. Just as a helper can operate machines and mix ingredients to create valuable products, potassium dichromate helps in creating important materials like dyes in the fabric industry and contributes to the leather-making process.
Key Concepts
-
Formation of Metal Oxides: Metals react with oxygen to form oxides at high temperatures, generally in ionic form.
-
Oxoanions: Anions that contain oxygen and a central metal, such as chromate and permanganate.
-
Disproportionation: A specific redox reaction where an element is both oxidized and reduced.
-
Solubility and Acidity: The oxides and oxoanions can demonstrate diverse behaviors such as acidity, basicity, and amphoteric properties.
Examples & Applications
Manganese in its +6 oxidation state can disproportionate to +7 and +4 states in acidic solutions.
Potassium dichromate is utilized as a strong oxidizing agent in various chemical reactions, particularly in the leather industry.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Oxides are formed with metals so bright, react with oxygen day or night.
Stories
Once in a lab, metals would meet oxygen at high temps, forming magical oxides, enduring as chemical gems.
Memory Tools
POTASSIUM - Permanganate, Oxidizer, Titration Agent, Strong Oxidizer, Sulfur.
Acronyms
CRUD - Chromate, Reduction, Oxidation, Dichromate.
Flash Cards
Glossary
- Oxides
Compounds formed by the reaction of metals with oxygen.
- Oxoanions
Anions that contain oxygen and a central metal atom.
- Disproportionation
The process where a single species is simultaneously oxidized and reduced.
- Chromate
An oxoanion containing chromium in the +6 oxidation state.
- Dichromate
An oxoanion containing chromium in the +6 oxidation state, commonly existing in acidic solutions.
- Permanganate
An oxoanion containing manganese in the +7 oxidation state, known for its strong oxidizing properties.
Reference links
Supplementary resources to enhance your learning experience.
