DIELECTRICS AND POLARISATION
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Dielectrics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning class! Today, we're diving into the world of dielectrics. Can anyone tell me what a dielectric is?

Isn't it a material that doesn't conduct electricity?

Exactly! Dielectrics are non-conductive materials. They don’t allow free movement of charge like conductors do. What happens when dielectrics are placed in an electric field?

They get polarized, right?

Correct! Polarization is a key concept here. So, polarization means that the molecules within the dielectric align their dipole moments to some extent. Why do you think this happens?

Maybe because the electric field tries to align the charges?

Absolutely! The external field influences the positive and negative charge centers in molecules, which can either create induced dipoles in non-polar molecules or align existing dipoles in polar molecules. Let's summarize: dielectrics are insulating materials that respond to electric fields primarily by polarization.
Polarization Mechanisms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

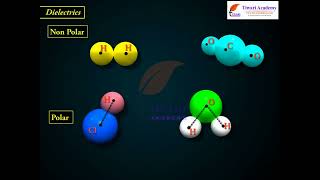
Now let's delve into the mechanisms of polarization. Can anyone highlight the difference between polar and non-polar molecules?

Polar molecules already have a dipole moment, while non-polar molecules develop one when subjected to an electric field.

Spot on! Polar molecules, like water, naturally have their positive and negative charges separated. Non-polar molecules, on the other hand, like oxygen, only induce a dipole moment when they are subjected to an electric field. What happens to the overall effect of polarization in these materials?

In non-polar materials, the dipoles are induced, while in polar materials, they align with the field!

Right again! This induced dipole moment in non-polar dielectrics contributes to the overall polarization but does not completely cancel the external electric field. Instead, it reduces it.
Impact on Capacitance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How do you think the presence of dielectrics affects the capacitance of capacitors?

I think it increases the capacitance!

Correct! When a dielectric is introduced between capacitor plates, the capacitance increases because the effective electric field is reduced, which allows more charge to be stored. We express this with the dielectric constant K, understood as a factor by which capacitance increases from the vacuum value. What is the formula?

C = K * C0, where C0 is the capacitance in a vacuum!

Exactly! So, we see dielectrics play a crucial role in enhancing the capabilities of capacitors in storing electric energy.
Applications and Implications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone provide examples of how dielectrics are used in real life?

They are used in capacitors for electronics!

Also in insulators for power lines!

Great examples! Dielectrics are not only essential in capacitors but also in insulators, allowing safe and efficient energy transmission. Remember, the efficiency and performance of electrical devices often hinge on the choice of appropriate dielectric materials. Let's wrap up what we've covered today.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains how dielectrics, being non-conductive, respond to external electric fields by inducing dipoles within their structure. It contrasts the behavior of polar and non-polar molecules under these fields and the significance of polarization in modifying electric fields and capacitance in capacitors.
Detailed
Dielectrics and Polarisation
Dielectrics, by definition, are materials that do not conduct electricity, distinguishing them from conductors that contain free charge carriers. This section explores how dielectrics react in the presence of external electric fields. When a dielectric is exposed to such an electric field, it does not provide the same response as a conductor.
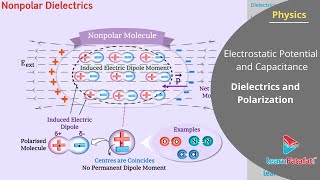
While conductors allow free movement of charge carriers (which redistribute and create an opposing electric field until internal electrostatic equilibrium is achieved), dielectrics induce a dipole moment. In non-polar molecules, the centers of positive and negative charges coincide under normal conditions; however, when exposed to an electric field, these charges shift, leading to the formation of an induced dipole moment.
In contrast, polar molecules inherently possess a dipole moment due to their asymmetrical charge distribution. The section emphasizes that the polarization effect only partially counteracts the external field, resulting in a reduced effective field within the dielectric, a crucial concept in understanding capacitors filled with dielectric materials. The relationship between the polarization of the dielectric and the external electric field is captured by the electric susceptibility, characterized by the equation P = e_0 * e * E, where P is the polarization, e is the permeability, and E is the electric field. Further, it discusses how these properties impact capacitance and the implications for designing capacitors.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Dielectrics
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Dielectrics are non-conducting substances. In contrast to conductors, they have no (or negligible number of) charge carriers. Recall from Section 2.9 what happens when a conductor is placed in an external electric field. The free charge carriers move and charge distribution in the conductor adjusts itself in such a way that the electric field due to induced charges opposes the external field within the conductor. This happens until, in the static situation, the two fields cancel each other and the net electrostatic field in the conductor is zero. In a dielectric, this free movement of charges is not possible.
Detailed Explanation
Dielectrics are materials that do not conduct electricity because they lack free moving charge carriers. This is different from conductors, where free electrons can move in response to electric fields. When a dielectric is placed in an external electric field, the charges within the dielectric do not move freely. Instead, the molecules of the dielectric begin to reorient or stretch, leading to induced dipole moments. However, the induced dipoles do not completely counteract the external electric field, unlike in conductors where the internal field becomes zero.
Examples & Analogies
Imagine putting a balloon (dielectric) in a room filled with people (electric field). The people can't move around freely, just as the charges in a dielectric cannot. Instead, the people might lean towards the balloon and create a slight pressure, similar to induced dipoles forming in the dielectric. The balloon does not push all the people away, just alters their positions slightly, thereby producing a weaker overall field inside the balloon.
Polarisation Process
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In an external electric field, the positive and negative charges of a non-polar molecule are displaced in opposite directions. The displacement stops when the external force on the constituent charges of the molecule is balanced by the restoring force (due to internal fields in the molecule). The non-polar molecule thus develops an induced dipole moment. The dielectric is said to be polarised by the external field.
Detailed Explanation
When a non-polar molecule is exposed to an external electric field, its symmetrical charge distribution is altered. The positive and negative charges are displaced slightly, leading to a separation of charge within the molecule which creates an induced dipole moment. This process continues until the internal forces within the molecule counterbalance the external field's forces. The net effect is that the dielectric material behaves as if it has a dipole moment in the direction of the field.
Examples & Analogies
Think of a rubber band (the molecule) stretched by two hands (the electric field). Initially, the rubber band is evenly stretched but when you pull on it, one side stretches more than the other, creating tension (induced dipole moment). This tension stabilizes once you stop pulling. The rubber band now exhibits a different shape due to the pulling forces, analogous to how molecules realign in a dielectric.
Effects of External Electric Field on Polar Molecules
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A dielectric with polar molecules also develops a net dipole moment in an external field. In the absence of any external field, the different permanent dipoles are oriented randomly due to thermal agitation; so the total dipole moment is zero. When an external field is applied, the individual dipole moments tend to align with the field.
Detailed Explanation
Polar molecules have permanent dipole moments, meaning they inherently have positive and negative charge centers that are separated. In a neutral state, these dipoles point in random directions, resulting in no net dipole moment. However, when subjected to an external electric field, these dipoles tend to align themselves along the direction of the field, enhancing the overall dipole moment of the dielectric.
Examples & Analogies
Think of a room full of people waving flags (the dipoles). Without any instruction, the flags are pointed in random directions (no net dipole moment). But if someone calls everyone to align their flags to point north (the electric field), you’ll end up with an organized and directional focus towards the north, thus creating a collective influence that is greater than when they were disorganized.
Polarisation and Electric Field Reduction
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus in either case, whether polar or non-polar, a dielectric develops a net dipole moment in the presence of an external field. The dipole moment per unit volume is called polarisation and is denoted by P. For linear isotropic dielectrics, P = ε0χE, where χ is the electric susceptibility of the dielectric medium.
Detailed Explanation
Under the influence of an electric field, both polar and non-polar dielectrics polarise, giving rise to net dipole moments which correlate with the strength of the external field. The amount of induced dipole moment per unit volume is defined as the polarisation (P). For specific dielectrics that respond linearly to electric fields, the polarisation is a product of the electric susceptibility (χ) of the material and the applied electric field (E). This relationship encapsulates how effectively a dielectric material can be polarized.
Examples & Analogies
Picture a sponge (the dielectric) being soaked in water (the electric field). The sponge absorbs water increasingly with more pressure applied. The amount of water absorbed at a given pressure is akin to polarisation, and the sponge's tendancy to hold and structure the water is akin to electric susceptibility. The more porous the sponge (higher χ), the more water it can absorb for the same pressure.
Dielectrics in Electric Fields
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The polarised dielectric is equivalent to two charged surfaces with induced surface charge densities, say σp and –σp. Clearly, the field produced by these surface charges opposes the external field. The total field in the dielectric is thus reduced from the case when no dielectric is present.
Detailed Explanation
When a dielectric material is polarized, it effectively creates two surfaces with induced charge densities on opposite sides. This results in a secondary electric field that opposes the original external field. The net effect is that the overall electric field inside the dielectric is weaker than outside. The extent of this reduction is determined by both the properties of the dielectric and the strength of the external electric field.
Examples & Analogies
Imagine trying to blow up a balloon (external field) inside a room with two windows open (dielectric). The air escaping from the windows (induced charges) makes it harder to fully inflate the balloon as the air escapes, reducing the effective pressure inside. This reflects how the presence of a dielectric reduces the effectiveness of the external electric field.
Key Concepts
-
Dielectric: A material that does not conduct electricity and can be polarized.
-
Polarization: The process of acquiring dipole moment in a dielectric when subjected to an electric field.
-
Polar Molecules: Molecules that have a permanent dipole moment.
-
Non-Polar Molecules: Molecules that develop a dipole moment when influenced by an electric field.
-
Electric Susceptibility: A measure of how susceptible a dielectric is to becoming polarized.
-
Capacitance: Defined as the charge stored per unit potential difference.
Examples & Applications
Water is a polar molecule, exhibiting significant interaction with electric fields.
Air-filled capacitors versus capacitor filled with a dielectric material, such as ceramic, show different capacitance levels.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Dielectric insulate, in fields they oscillate.
Stories
Imagine molecules at a party. Polar molecules hold hands (dipole), while non-polar just sit until invited (polarized).
Memory Tools
Remember 'PANDA' - Polarization And Non-Polar molecules Align.
Acronyms
DICE - Dielectrics Induce Charge Electrical fields.
Flash Cards
Glossary
- Dielectric
A dielectric is a substance that does not conduct electricity and can be polarized in an electric field.
- Polarization
The process in which a dielectric material acquires an induced dipole moment in response to an electric field.
- Polar Molecule
A molecule that has a permanent dipole moment due to the unequal distribution of charges.
- NonPolar Molecule
A molecule that does not have a permanent dipole moment and becomes polarized under the influence of an electric field.
- Electric Susceptibility
A measure of how easily a material can be polarized by an electric field.
- Capacitance
The ability of a body to store electric charge; quantitatively defined as the charge per unit potential difference.
Reference links
Supplementary resources to enhance your learning experience.
