Effect of frequency of incident radiation on stopping potential
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Stopping Potential
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore stopping potential in the context of the photoelectric effect. Can anyone explain what we mean by 'stopping potential'?

Is it the minimum voltage needed to stop electrons from reaching the collector plate?

Exactly, well done! The stopping potential is indeed the critical value that opposes the motion of the most energetic photoelectrons. Now, can anyone tell me how this relates to the frequency of the light used?

I think it changes based on the frequency. Higher frequencies mean more energy.

That's correct! The kinetic energy of the ejected electrons increases with the frequency of incident light, thereby requiring a higher stopping potential to halt these electrons. Remember this key point: 'Frequency Up, Stopping Potential Up!'
Linear Relationship Between Frequency and Stopping Potential
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Okay, now let’s discuss the relationship between frequency and stopping potential. Who can summarize this relationship for me?

The stopping potential increases linearly with frequency, right?

Absolutely! This linearity means that as you increase the frequency of the incident light, the stopping potential needed to halt the electrons also increases. If we plot this, we get a straight line. How does this compare to the effect of intensity?

Intensity doesn’t change the stopping potential; it only affects the number of electrons emitted, not their energy.

Exactly! So, remember: 'Intensity affects quantity, frequency affects quality!' Great job!
Threshold Frequency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s now shift our focus to the concept of threshold frequency. Who can explain what this means?

It’s the minimum frequency needed for photoelectric emission to occur, right?

Exactly! If the frequency of light is lower than this threshold frequency, no photoelectrons will be emitted regardless of how intense the light is. How would you find the threshold frequency for different materials?

We would need to look at the work function for those materials, then use the equation to find it.

Great point! Always connect the stopping potential to the threshold frequency via the work function. Keep in mind: 'Threshold Frequency = Key for Emission!'
Kinetic Energy of Photoelectrons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know about stopping potential and threshold frequency, how do these concepts inform us about the kinetic energy of photoelectrons?

The kinetic energy should increase with frequency, right? It’s related to the stopping potential.

Correct! The maximum kinetic energy of emitted photoelectrons increases as the frequency increases, and it is given as K_max = eV₀. This means the stopping potential directly represents the energy needed to stop these electrons.

So, higher frequency not only increases stopping potential but also maximum kinetic energy?

Exactly! Summarizing: 'More Frequency = More Energy!' Remember, this is a critical concept in understanding the photoelectric effect.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains that as the frequency of the incident radiation increases, the stopping potential also increases, indicating that the maximum kinetic energy of emitted electrons is directly related to the frequency of the radiation rather than its intensity.
Detailed
In this section, we explore the relationship between the frequency of incident radiation and the stopping potential (V₀) necessary to halt photoelectric emission from a metal surface. It is established that the energy of emitted electrons is dependent on the frequency of radiation, with higher frequencies resulting in higher stopping potentials. Detailed observations indicate that the stopping potential varies linearly with frequency, while remaining independent of intensity. This leads to the conclusion that the maximum kinetic energy of photoelectrons correlates directly with frequency, as given by the formula K_max = eV₀, which conforms to the findings of the photoelectric effect. Furthermore, a threshold frequency exists for each material, below which no electrons can be emitted, regardless of the intensity of incident light.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Variation of Stopping Potential with Frequency
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
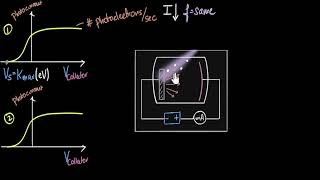
We now study the relation between the frequency n of the incident radiation and the stopping potential V₀. We suitably adjust the same intensity of light radiation at various frequencies and study the variation of photocurrent with collector plate potential. The resulting variation is shown in Fig. 11.4. We obtain different values of stopping potential but the same value of the saturation current for incident radiation of different frequencies. The energy of the emitted electrons depends on the frequency of the incident radiations. The stopping potential is more negative for higher frequencies of incident radiation.
Detailed Explanation
This chunk explains how the stopping potential (V₀) of photoelectrons varies with the frequency (n) of the incident radiation. As the frequency increases, the energy that electrons gain also increases, and higher stopping potentials are needed to prevent these more energetic electrons from reaching the collector plate. This means that if we shine light at different frequencies but with the same intensity, we will get different stopping potentials.
Examples & Analogies
Imagine a game where you are kicking balls at a target—it’s easier to stop a slow ball than a faster one. Similarly, the photoelectrons ejected by light at a higher frequency have more energy (like the fast balls), and therefore, require a stronger opposing force (higher stopping potential) to stop them.
Linear Relationship Between Frequency and Stopping Potential
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Note from Fig. 11.4 that the stopping potentials are in the order V₀₃ > V₀₂ > V₀₁ if the frequencies are in the order n₃ > n₂ > n₁. This implies that greater the frequency of incident light, greater is the maximum kinetic energy of the photoelectrons. Consequently, we need greater retarding potential to stop them completely. If we plot a graph between the frequency of incident radiation and the corresponding stopping potential for different metals we get a straight line.
Detailed Explanation
The stopping potential not only increases with frequency, but this increase is linear. This means that if you were to graph these two variables, you'd get a straight line, indicating a direct relationship. The greater the frequency of the incoming light, the more energy the electrons have, thus requiring a higher stopping potential to prevent them from passing through.
Examples & Analogies
Think of it like the amount of force needed to stop a car. If the car is going faster (higher frequency), more force (higher stopping potential) is required to halt its movement. The straight line on a graph represents the consistent relationship between the speed of the car (frequency) and the power required to stop it.
Threshold Frequency and Photoelectric Emission
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These observations have two implications: (i) The maximum kinetic energy of the photoelectrons varies linearly with the frequency of incident radiation, but is independent of its intensity. (ii) For a frequency n₀ of incident radiation, lower than the cut-off frequency n₀, no photoelectric emission is possible even if the intensity is large. This minimum, cut-off frequency n₀ is called the threshold frequency. It is different for different metals.
Detailed Explanation
The threshold frequency is critical because it is the minimum frequency needed for photoelectric emission to occur. Below this frequency, no matter how much light is shining on the metal, electrons will not be ejected. This establishes two important points: the kinetic energy of the emitted electrons is related to the frequency of the light, and there is a limit (the threshold frequency) below which no emission occurs regardless of light intensity.
Examples & Analogies
Consider a door that only opens if you push with enough force. The threshold frequency is like the minimum push needed to make the door swing open. If you don’t push hard enough (lower frequency), it stays shut (no electron emission), regardless of how much you press (light intensity).
Instantaneous Photoelectric Emission
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Note that in all the above experiments, it is found that, if frequency of the incident radiation exceeds the threshold frequency, the photoelectric emission starts instantaneously without any apparent time lag, even if the incident radiation is very dim.
Detailed Explanation
This chunk addresses how quickly photoelectrons are emitted once the incident light frequency surpasses the threshold frequency. It emphasizes that the emission is almost instantaneous, signifying that this process occurs swiftly as the electrons absorb energy from the photons in the light.
Examples & Analogies
Imagine a light switch that turns on instantly when you flip it. The moment you flip the switch (reach the threshold), the light comes on (electrons are emitted) without delay. This highlights how light can have immediate effects upon interacting with the right materials.
Key Concepts
-
Stopping Potential: The voltage needed to stop the most energetic photoelectrons.
-
Threshold Frequency: The minimum frequency required for photoelectric emission.
-
Kinetic Energy of Photoelectrons: The energy of emitted electrons relates directly to the frequency, not intensity.
-
Linearity of Stopping Potential and Frequency: Stopping potential increases linearly with increasing frequency.
Examples & Applications
Consider a metal with a threshold frequency of 5 x 10^14 Hz. If light with a frequency of 7 x 10^14 Hz is applied, photoemission occurs, and the stopping potential can be calculated.
Using a stopping potential of 1.2 V for a frequency of incident radiation of 6 x 10^14 Hz, we can determine the maximum kinetic energy of electrons ejected.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For emission in the light so bright, frequency is key, keeping electrons in flight.
Stories
Imagine a race: the higher the frequency of the light, the faster the electrons can run away. But if the light doesn't meet the threshold, they’re stuck, unable to leave.
Memory Tools
F-S-K: Frequency increases Stopping potential and Kinetic energy.
Acronyms
SFT
Stopping Potential
Frequency
Threshold - remember these for photoelectric effect.
Flash Cards
Glossary
- Stopping Potential (V₀)
The minimum potential needed to stop the most energetic photoelectrons from reaching the collector plate.
- Threshold Frequency (n₀)
The minimum frequency of incident light required to emit photoelectrons from a material.
- Kinetic Energy (K_max)
The maximum energy of emitted photoelectrons, which is determined by the frequency of the incident radiation.
- Photoelectric Effect
The emission of electrons from a material when it is illuminated by light of sufficient frequency.
- Work Function (f₀)
The minimum energy needed for an electron to escape from the surface of a metal.
Reference links
Supplementary resources to enhance your learning experience.
