EINSTEIN’S PHOTOELECTRIC EQUATION: ENERGY QUANTUM OF RADIATION
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Photoelectric Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're exploring the photoelectric effect, which describes how light can cause the emission of electrons from a material when the light hits its surface.

Was this always understood? How did we come to know about it?

Good question! The photoelectric effect was discovered in 1887 by Heinrich Hertz during his experiments with electromagnetic waves. He noticed that the emission of electrons from certain metals is dependent on the light frequency.

Does this mean light behaves like a particle?

Exactly! This particle-like behavior of light was solidified by Einstein in 1905.
Einstein's Contribution
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Einstein proposed that light consists of particles called photons, each with energy given by E = hn, where h is Planck's constant.

How does this relate to the electrons being emitted?

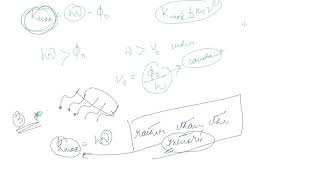
Upon absorbing a photon with sufficient energy, an electron can escape from the metal's surface. The maximum kinetic energy of the emitted electron is K_max = hn - φ₀.

What happens if the photon energy is less than the work function?

If the energy is insufficient, the electron will not be released. This introduces the important concept of threshold frequency.
Threshold Frequency and Work Function
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss threshold frequency. For each metal, there’s a minimum frequency below which no photoemission occurs, despite the intensity of light.

So it doesn't matter how bright the light is, if the frequency isn't right?

Right! The energy from the photons must exceed the work function, φ₀, to free an electron from the metal's attraction.

How does this relate to practical applications?

Great point! The principles from the photoelectric effect are integral to technologies such as solar panels and photodetectors.
Instantaneous Emission
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

A unique feature of the photoelectric effect is that electron emission occurs almost instantaneously, typically within 10⁻⁹ seconds.

What does this mean for the light intensity?

The intensity of light affects the number of electrons emitted but not their individual kinetic energy—a powerful concept!

This sounds like the presence of discrete energy levels!

Exactly! Just as in atomic structures, energy is quantized within the photons.
Conclusion and Implications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap it up, Einstein's photoelectric equation was groundbreaking. It challenges classical physics by introducing the photon concept.

What was the broader impact of this?

Einstein's work laid the foundation for quantum mechanics, fundamentally changing our understanding of light and matter interaction.

Can we see this in other experiments?

Absolutely! Similar principles apply in the Compton Effect and other quantum phenomena.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In 1905, Einstein introduced a new understanding of electromagnetic radiation, establishing that radiation energy is composed of discrete units called quanta. His photoelectric equation relates the energy of photons to the emission of electrons from metals, showing the dependency on frequency and not intensity, presenting a solution to previously unexplained phenomena observed in photoelectric experiments.
Detailed
Einstein’s Photoelectric Equation
In 1905, Albert Einstein proposed a revolutionary view on electromagnetic radiation, introducing the concept of quanta, or photons, which carry discrete units of energy. According to his formulation, the energy of a photon is expressed as E = hn, where h is Planck’s constant and n is the frequency of light. This theory effectively describes the photoelectric effect—the phenomenon where light causes the emission of electrons from a metal surface.
The essence of Einstein’s equation is captured in the relation:
- K_max = hn - φ₀
Where:
- K_max is the maximum kinetic energy of the emitted electrons,
- φ₀ is the work function of the metal (the minimum energy required to release an electron).
This equation outlines two critical aspects: the emission of electrons only occurs when the energy of the photon (hn) exceeds the work function (φ₀), hence introducing the concept of a threshold frequency, below which no emission occurs regardless of light intensity. Additionally, if the energy exceeds the work function, the kinetic energy of the emitted electrons is determined by the excess energy above the work function.
Overall, Einstein's work not only elucidated the photoelectric effect but also laid a foundation for the development of quantum mechanics, demonstrating the dual nature of light as both a wave and a particle.
Youtube Videos






Key Concepts
-
Work Function: The minimum energy required to release an electron from the metal's surface.
-
Threshold Frequency: The minimum frequency required for the photoelectric effect to occur.
-
Einstein's Photoelectric Equation: K_max = hn - φ₀, describes the maximum kinetic energy of emitted electrons.
Examples & Applications
If a metal has a work function of 2.14 eV, light with a frequency exceeding its threshold will emit electrons.
When ultraviolet light strikes cesium, electrons are emitted due to sufficient photon energy fitting the requirements set by Einstein's equation.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If light is bright and frequency high, electrons will jump and fly!
Stories
Imagine a party at a metal surface. Photons are guests—only those with knack (sufficient energy) can dance (free electrons) and leave the party!
Memory Tools
P.E. = Photon Energy = hn. Remember, higher frequency means higher energy!
Acronyms
E=MC...Energy = Max Kinetic (K_max) + Work Function (φ₀)!
Flash Cards
Glossary
- Photoelectric Effect
The emission of electrons from a material when it is exposed to light of sufficient frequency.
- Photon
A quantum of electromagnetic radiation, representing a discrete packet of energy.
- Work Function (φ₀)
The minimum energy needed to release an electron from the surface of a material.
- Threshold Frequency
The minimum frequency of light needed to produce the photoelectric effect in a given material.
- Planck's Constant (h)
A fundamental physical constant that relates the energy of a photon to its frequency, approximately 6.63 × 10−34 Js.
Reference links
Supplementary resources to enhance your learning experience.
