Effect of intensity of light on photocurrent
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Photocurrent and Light Intensity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss how the intensity of light affects photocurrent. Let's start with what we know about photocurrent. Can anyone explain what photocurrent is?

Photocurrent is the electric current produced when light hits a photosensitive material and ejects electrons.

Exactly! Now, when we talk about light intensity, what do we mean?

I think it refers to how much light energy is coming from a source. Higher intensity means more energy.

That's right! The intensity of light is directly related to the number of photons emitted per second. Now, let's dive into how this intensity affects photocurrent. What do you guys think will happen to photocurrent if we increase the intensity of light while keeping the frequency constant?

I think the photocurrent would increase because more photons would hit the surface and potentially eject more electrons.

Absolutely! This leads us to a key point: the photocurrent is directly proportional to the light intensity, as depicted in a linear graph. This means that as intensity increases, so does the current. Great job thinking this through!
Understanding the Linear Relationship
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's explore why the relationship between intensity and photocurrent is linear. Can anyone summarize what this linearity implies?

This means that if we double the intensity, we should also double the photocurrent, right?

Spot on! This linearity demonstrates that the number of photoelectrons emitted per second depends directly on how many photons are incident on the metal surface. If there are more photons, more electrons can escape. How does this differ from what we might expect with classical wave theory?

The wave theory suggests that increasing intensity increases energy absorption continuously, not just the number of emitted electrons.

Exactly! The wave theory fails to explain the result since it implies that higher intensity would also mean higher energy per electron. In reality, the energy of each emitted electron varies based on frequency, not intensity. Keep these differences in mind as we move forward!
Experimental Observations and Implications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s briefly review the experimental setup used to measure these effects. Can anyone describe how the experiments are structured?

We have a photosensitive plate that emits electrons when illuminated, and we measure the current produced based on different light intensities.

Correct! And keeping the potential difference constant helps us focus solely on the effects of intensity and frequency. What results stand out from these experiments?

The photocurrent increases linearly with the intensity, confirming our earlier discussions.

Well summarized! This is a crucial discovery in confirming the particle nature of light as well, and sets the stage for understanding more complex phenomena like the photoelectric effect. Can't forget to note that if the frequency is below the threshold, no photocurrent occurs regardless of intensity. Great observations today!
Real-Life Applications and Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we conclude, let's talk about why understanding the intensity-photocurrent relationship is important. How can this be applied in real life?

It can be important in designing solar panels—knowing how much light intensity can maximize the output current.

Exactly! Such knowledge enables us to optimize energy conversion technologies and improve their efficiency. Thinking broader, this understanding shapes not just technology, but also theoretical physics. What are your thoughts about how this might affect future research?

It could help develop better electronic devices that harness light energy more effectively or even lead to new materials.

Fantastic insights! Remember, every advance in our grasp of light and its properties can open doors to innovations we can hardly envision today. Well done, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The intensity of light directly influences the photocurrent produced when light of fixed frequency illuminates a photosensitive material. As the intensity increases, the photocurrent increases linearly, showcasing how the number of photoelectrons emitted is contingent on the intensity of the incident radiation.
Detailed
Detailed Summary
The section discusses the impact of the intensity of light on the photocurrent generated during the photoelectric effect. When light of a certain frequency strikes a photosensitive plate, it ejects electrons, known as photoelectrons. The experiments show that by keeping the frequency of the light constant while varying its intensity, the resulting photocurrent increases linearly. This is because the photocurrent is directly proportional to the number of photoelectrons emitted per second. Higher intensity means more photons are hitting the metal surface, leading to a greater number of electrons being released. This linear relationship not only reaffirms the essential characteristics of the photoelectric effect but also highlights the dependence of photon emission on light intensity and the fundamental principles governing electron behavior in response to light.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Setup the Experiment
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
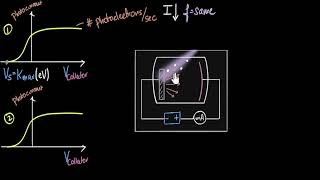
The collector A is maintained at a positive potential with respect to emitter C so that electrons ejected from C are attracted towards collector A. Keeping the frequency of the incident radiation and the potential fixed, the intensity of light is varied and the resulting photoelectric current is measured each time.
Detailed Explanation
In the study of the photoelectric effect, an experimental setup is used where one plate (collector A) has a positive potential compared to another plate (emitter C). This means that when light hits the emitter, it will knock out electrons, which are then attracted to the positively charged collector plate. By changing the intensity of the light while keeping everything else constant (like the frequency of the light and the voltage), we can observe how this affects the electric current generated due to the emitted electrons.
Examples & Analogies
Think of this as trying to pump water through a hose. If the percentage of water flowing (the intensity of light) increases while the configuration of the hose (the setup) remains unchanged, you can measure a corresponding increase in the water flowing out of the other end (photocurrent).
Relationship Between Photocurrent and Intensity
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is found that the photocurrent increases linearly with intensity of incident light as shown graphically in Fig. 11.2.
Detailed Explanation
The linear relationship between photocurrent and intensity of the light means that as the light gets brighter (increased intensity), the number of electrons ejected from the emitter and subsequently collected at the collector also increases proportionally. This indicates that more photons result in more ejected photoelectrons, leading to a higher current—this is a direct response to the change in intensity.
Examples & Analogies
Imagine using brighter and brighter flashlights in a dark room. The brighter the flashlight's beam (intensity), the more shadows you can see on the wall (photoelectrons being emitted), just as increasing the light intensity corresponds to a higher photocurrent.
Understanding the Proportionality
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The photocurrent is directly proportional to the number of photoelectrons emitted per second. This implies that the number of photoelectrons emitted per second is directly proportional to the intensity of incident radiation.
Detailed Explanation
Here, the core idea is that the photocurrent can be quantified in terms of how many electrons are being emitted from the emitter material per second. Since each photon that strikes the emitter can potentially knock out an electron, if you increase the number of photons hitting the surface (which happens with increased light intensity), you also increase the total number of electrons that are knocked out. Therefore, the more intense the light, the more electrons are emitted, directly increasing the photocurrent.
Examples & Analogies
Think of it like throwing balls at a target; if you throw more balls per second (high intensity), the chances of hitting the target (emitting electrons) increase, thereby increasing the results you see (the photocurrent measured).
Key Concepts
-
Photocurrent: The current produced by ejected electrons when light strikes a photosensitive surface.
-
Intensity of Light: Refers to how much light energy is available, affecting the number of emitted electrons.
-
Linear Relationship: The photocurrent increases proportionally with the intensity of light, demonstrating direct proportionality.
-
Threshold Frequency: The minimum frequency of light needed to produce photocurrent; below this, no current is produced.
Examples & Applications
When a zinc plate is illuminated with ultraviolet light, its photoelectric current increases with higher light intensity, demonstrating the linear relationship.
If the intensity of light hitting a metal is doubled, the rate of emitted photoelectrons also doubles, resulting in double the photocurrent.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For photocurrent to rise, intensity is key, double the light, double the glee!
Stories
Imagine a factory where workers (photoelectrons) can only leave (get ejected) when the lights (light intensity) are bright enough. If the lights are too dim, no one can leave!
Memory Tools
I-P-E (Intensity, Photocurrent, Ejected electrons) to remember the relationships.
Acronyms
P.I.L. - Photocurrent Increases with Light intensity.
Flash Cards
Glossary
- Photocurrent
The electric current generated as a result of the emission of electrons when light strikes a photosensitive material.
- Intensity of Light
The power per unit area carried by a wave, typically correlated with the energy carried by photons in a light wave.
- Photoelectron
An electron that is emitted as a result of the photoelectric effect when light shines on a material.
- Threshold Frequency
The minimum frequency of light required to eject electrons from a certain material.
Reference links
Supplementary resources to enhance your learning experience.
