Hallwachs’ and Lenard’s observations
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Photoelectric Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re diving into photoelectric emission, the process where light causes the release of electrons from a material. Can anyone name the physicists who were key in this discovery?

Was it Hallwachs and Lenard?

Correct! They explored how ultraviolet radiation impacts metal plates. Lenard noticed that current only flowed when the ultraviolet light was present. Why do you think that is?

Because the light must be exciting the electrons to escape the metal?

Exactly! This connected light directly to the electron emission process.
Hallwachs' Contributions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Hallwachs made observations regarding zinc plates. He connected a negatively charged zinc plate to an electroscope and found that the charge decreased with ultraviolet exposure. Can someone explain why this indicates the emission of electrons?

When the plate loses its negative charge, it must mean that electrons are leaving the plate due to the ultraviolet light.

Exactly! The emitted electrons were termed 'photoelectrons.' Let’s remember that, the term 'photoelectron' signifies electrons released due to light.
Lenard's Findings
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lenard’s findings were crucial. He observed that current only flows when ultraviolet light hits the plate, disappearing without it. What does that tell us about the relationship between light and electrons?

It shows that some minimum energy, provided by light, is required to release electrons.

Correct again! This minimum light intensity links to the threshold frequency, a key concept for us to understand.
Threshold Frequency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s think about threshold frequency. Lenard and Hallwachs found that different metals react differently. Can anyone give an example of metals and their light frequencies?

Zinc only reacts to ultraviolet light, while alkali metals can react to visible light as well.

Good observation! Metals have their unique thresholds for electron emission depending on their properties.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
During the late 19th to early 20th centuries, Hallwachs and Lenard explored the photoelectric effect. Their findings demonstrated that ultraviolet light could cause electron emission from metals, emphasizing the relationship between light frequency and electron behavior. These observations were pivotal in validating the quantum nature of light.
Detailed
Hallwachs’ and Lenard’s Observations
Wilhelm Hallwachs and Philipp Lenard were instrumental in exploring the phenomenon of photoelectric emission between 1886 and 1902. Lenard discovered that when ultraviolet radiation was directed at a metal plate in a vacuum tube containing electrodes, an electric current flowed. This current ceased immediately when the radiation was removed, indicating a direct link between light exposure and electron emission.
Hallwachs expanded on these findings in 1888 by connecting a negatively charged zinc plate to an electroscope. He noted that upon exposure to ultraviolet light, the zinc plate lost its charge and then became positively charged with further illumination. This led him to conclude that negatively charged particles, later known as electrons, were emitted from the zinc due to the interaction with ultraviolet light.
Both researchers observed a critical threshold frequency for electron emission, underscoring that different materials have distinct sensitivity to light frequencies. Metals like zinc only responded to ultraviolet light, while others, such as alkali metals, could emit electrons when exposed to visible light. Their pioneering work laid the foundation for understanding the quantum nature of light and supported the eventual formulation of the photoelectric effect.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Initial Observations by Lenard
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
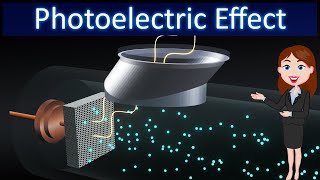
Lenard (1862-1947) observed that when ultraviolet radiations were allowed to fall on the emitter plate of an evacuated glass tube enclosing two electrodes (metal plates), current flows in the circuit (Fig. 11.1). As soon as the ultraviolet radiations were stopped, the current flow also stopped. These observations indicate that when ultraviolet radiations fall on the emitter plate C, electrons are ejected from it which are attracted towards the positive, collector plate A by the electric field.
Detailed Explanation
Lenard's experiments showed that ultraviolet light interacts with a specific metal plate (the emitter plate). When this light hits the plate, it provides energy to electrons, allowing them to overcome the attractive forces holding them within the metal. As a result, these electrons are ejected and flow towards a positively charged plate, creating an electric current. When the light is turned off, this ejected current stops, indicating a direct relationship between the presence of light and electron emission.
Examples & Analogies
Imagine a rubber band that holds marbles (electrons) tightly together. When you shine a light on the band, it gives energy to the marbles, allowing some to break free and roll towards a bucket (the collector plate). The moment you turn off the light, the marbles cannot escape anymore, and the flow of marbles stops.
Hallwachs’ Further Investigations
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hallwachs, in 1888, undertook the study further and connected a negatively charged zinc plate to an electroscope. He observed that the zinc plate lost its charge when it was illuminated by ultraviolet light. Further, the uncharged zinc plate became positively charged when it was irradiated by ultraviolet light.
Detailed Explanation
Hallwachs built upon Lenard's findings by conducting experiments with a zinc plate connected to an electroscope, which measures electrical charge. He found that shining ultraviolet light on the zinc plate caused it to release negatively charged particles (electrons), leading to a loss of negative charge on the plate, hence resulting in a net positive charge. This demonstrated that light not only made current flow but also actively affected the charge state of materials.
Examples & Analogies
Think of charging a balloon by rubbing it against your hair. When you rub the balloon (like shining ultraviolet light on the zinc plate), it collects negative charges (electrons), making it negative. If you leave it in the air, it can lose its charge (just as the zinc plate loses electrons) and induce a positive charge nearby.
Threshold Frequency and Material Response
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hallwachs and Lenard also observed that when ultraviolet light fell on the emitter plate, no electrons were emitted at all when the frequency of the incident light was smaller than a certain minimum value, called the threshold frequency. This minimum frequency depends on the nature of the material of the emitter plate.
Detailed Explanation
They found that each material has a specific frequency of light below which it cannot emit electrons. This is known as the threshold frequency. If the frequency of the incident light is lower than this threshold, the energy provided is insufficient to free the electrons from their binding within the metal, resulting in no emission, regardless of light intensity. This concept highlights that not all light can cause photoelectric emission; only specific frequencies can.
Examples & Analogies
Consider a seed that requires a specific amount of sunlight to sprout. If it's placed in a dark room (lower frequency), it won't sprout no matter how long it stays there. But with enough sunlight (above a threshold), it will start to grow and break through the soil, mirroring the concept of frequency being the key to releasing electrons.
Sensitivity of Different Metals
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It was found that certain metals like zinc, cadmium, magnesium, etc., responded only to ultraviolet light, having short wavelength, to cause electron emission from the surface. However, some alkali metals such as lithium, sodium, potassium, caesium and rubidium were sensitive even to visible light.
Detailed Explanation
Different materials respond differently to light. While metals like zinc require ultraviolet light to emit electrons, alkali metals can emit electrons when exposed to visible light. This reveals that not only the type of light but also the material's properties play a significant role in the photoelectric effect, influencing their sensitivity to different parts of the light spectrum.
Examples & Analogies
Think of a variety of plants that need different amounts of sunlight. Some plants (like zinc) thrive with bright sunlight (ultraviolet light), while others (like alkali metals) can grow well even in partial shade (visible light). Each one has a unique set of requirements for growth, just as different metals have different threshold light wavelengths.
Photoelectric Effect Identification
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
All these photosensitive substances emit electrons when they are illuminated by light. After the discovery of electrons, these electrons were termed as photoelectrons. The phenomenon is called photoelectric effect.
Detailed Explanation
The culmination of Hallwachs and Lenard’s work led to the identification of the photoelectric effect, where certain substances emit electrons (photoelectrons) when illuminated by light. The understanding that light could induce electron emission formed a foundational idea in quantum mechanics and the study of light's particle nature.
Examples & Analogies
It's similar to how a floor is made slippery when a water balloon bursts on it. Just like the balloons cause changes to their environment, the interaction of light with sensitive metals causes a reaction that results in the emission of electrons, changing the state of the metal.
Key Concepts
-
Photoelectric Effect: A process in which electrons are emitted from a material when light shines upon it.
-
Threshold Frequency: Each material has a certain minimum frequency of light needed to emit electrons.
-
Hallwachs and Lenard: Key scientists who significantly contributed to understanding the photoelectric effect.
Examples & Applications
When ultraviolet light is shone on a zinc plate, electrons are released, demonstrating the photoelectric effect.
Different metals require varying frequencies of light; zinc does not respond to visible light while alkali metals do.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In light of UV rays so bright, electrons fly out of sight!
Stories
Imagine zinc at a party where UV light is the DJ; when the music plays, the electrons get excited and dance away!
Memory Tools
Remember 'PEC' for Photoelectric Effect and Current: Photoelectric Effect leads to emitted Current.
Acronyms
HLP
Hallwachs and Lenard study Photoelectric effect.
Flash Cards
Glossary
- Photoelectric Effect
The phenomenon where electrons are emitted from a material when exposed to light.
- Threshold Frequency
The minimum frequency of light required to emit electrons from a metal surface.
- Photoelectron
Electrons that are emitted from a material due to exposure to light.
Reference links
Supplementary resources to enhance your learning experience.
