Corrosion Inhibitors
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Corrosion Inhibitors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss corrosion inhibitors. Can anyone tell me why we need such additives in concrete?

I think they help protect the steel inside the concrete from rusting.

Exactly! Corrosion inhibitors protect reinforcement bars from corrosion. They can work in two ways: by forming a protective film or increasing the pH of the concrete.

What types of corrosion inhibitors are there?

Great question! Two common examples are calcium nitrite and sodium benzoate. Let’s remember this as 'CS' for Corrosion Solutions!

Where are these used?

They are used in marine structures, parking garages, and bridges, where corrosion risk is higher due to exposure to moisture and de-icing agents.

So, it's mostly about durability?

Yes! Enhancing durability is key. Let's summarize: corrosion inhibitors protect steel by forming a barrier and elevating pH, used in critical structures.
Mechanism of Action
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s dive deeper into how these inhibitors work. Can someone explain what happens to the steel when corrosion starts?

Corrosion happens when the steel reacts with oxygen and moisture, right?

Right! Corrosion inhibitors can prevent this by either increasing the concrete’s pH or forming a protective film. Who can explain how this film works?

It acts like a barrier that stops the moisture from reaching the steel?

Exactly! This barrier prevents oxidative reactions on the steel surface. Remember, it’s like how rain jackets keep us dry in the rain—keeping the vulnerable part protected.

And the pH increase helps too?

Yes! A higher pH creates an environment where the steel naturally forms a protective oxide layer, making it resistant to corrosion. Let's recap: protective films and elevated pH are the key mechanisms.
Real-World Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s connect theory to practice. Where do you think corrosion inhibitors would be essential?

In bridges, because they get a lot of water exposure.

Correct! Bridges are a prime example. Other structures include marine environments like docks or piers. Why do you think that is?

Because they face saltwater?

Absolutely! Saline environments accelerate corrosion. So, using corrosion inhibitors in these locations helps extend the lifespan of the structure.

What kind of inhibitors would be used?

Calcium nitrite is very common in these applications. Remember: marine and parking structures need extra protection.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
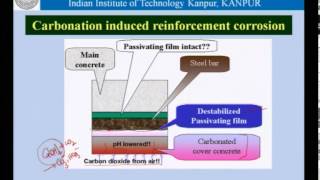
Corrosion inhibitors are chemical admixtures used in concrete to protect steel reinforcement from corrosion, particularly in harsh environments. They work by forming a protective film on the steel surface or increasing the pH of the concrete mix, enhancing durability and longevity in structures such as bridges and marine installations.
Detailed
Corrosion Inhibitors
Corrosion inhibitors are vital chemical admixtures added to concrete that serve to protect the embedded steel reinforcement from corrosion. This corrosion can significantly compromise the structural integrity and longevity of concrete structures, especially those exposed to aggressive environmental conditions like marine atmospheres or de-icing salts.
Purpose
Corrosion inhibitors primarily aim to safeguard the reinforced concrete by either:
- Forming a protective film on the surface of the exposed rebar (reinforcement bars).
- Increasing the pH level of the concrete surround, which aids in maintaining the passivation layer on the steel to resist corrosion processes.
Mechanism
These inhibitors typically work via various chemical pathways to enhance the durability of concrete structures by minimizing the risk of corrosion. For instance, the formation of a protective film helps to isolate the steel from corrosive agents, while elevated pH levels can prevent the onset of corrosion reactions (like oxidation).
Examples and Applications
Common examples of corrosion inhibitors include:
- Calcium Nitrite: Known to enhance the corrosion resistance of reinforcing steel. It is widely used in marine structures, bridges, and parking garages where corrosion risk is high.
- Sodium Benzoate: This is another inhibitor that improves the overall resistance of concrete to corrosion.
Corrosion inhibitors find extensive applications in structures that are particularly vulnerable to environmental challenges, including marine structures, bridges, and parking garages, where the potential exposure to chlorides and moisture is a concern.
In summary, the utilization of corrosion inhibitors in concrete technology plays a crucial role in enhancing the service life of structures in aggressive environments.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Purpose of Corrosion Inhibitors
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Protect reinforcement from corrosion.
Detailed Explanation
Corrosion inhibitors are added to concrete to safeguard the steel reinforcement bars (rebars) from rusting and degradation. Rusting occurs when steel is exposed to moisture and oxygen, leading to the formation of iron oxide (rust) which ultimately weakens the concrete structure. Corrosion inhibitors, therefore, play a crucial role in enhancing the longevity and durability of concrete structures by preventing this corrosion process.
Examples & Analogies
Imagine a metal bicycle left outside in the rain. Without protection, it rusts and weakens over time. Similarly, rebars in concrete need protection from moisture and other elements to maintain their strength, just as you would cover your bicycle with a tarp or bring it indoors to prevent it from rusting.
Mechanism of Action
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Form protective film on rebars or increase pH.
Detailed Explanation
Corrosion inhibitors work through several mechanisms. One way is by forming a protective film on the surface of the rebars. This film acts as a barrier, preventing moisture and oxygen from reaching the steel. Another mechanism is increasing the pH level of the concrete environment, which makes it more alkaline. High pH levels can help maintain a passive layer on the steel, further preventing corrosion.
Examples & Analogies
Think of corrosion inhibitors like a raincoat for your metal bike. Just as a raincoat keeps water away from the bike, a protective film keeps moisture away from the steel in concrete. Additionally, raising the pH of the surrounding environment can be likened to keeping the bike in a dry, sheltered spot, which slows down deterioration.
Examples of Corrosion Inhibitors
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Calcium nitrite, sodium benzoate.
Detailed Explanation
Several substances serve as effective corrosion inhibitors. Calcium nitrite and sodium benzoate are among the most commonly used. Calcium nitrite not only protects steel from corrosion but also enhances the hydration of concrete. Sodium benzoate, on the other hand, helps to boost the concrete's resistance to chloride-induced corrosion. Both these compounds are purposeful in reinforcing the durability of structures where steel is present.
Examples & Analogies
Imagine calcium nitrite as a friend who helps you study better and stay organized; it not only protects you but also enhances your performance. Sodium benzoate can be seen as a sport's equipment that enhances your game, making it safer from unexpected challenges such as environmental effects.
Applications of Corrosion Inhibitors
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Marine structures, parking garages, bridges.
Detailed Explanation
Corrosion inhibitors are particularly essential in environments where concrete is exposed to harsh conditions. For instance, marine structures face salty seawater which can accelerate corrosion. Parking garages are often subjected to de-icing salts in winter, contributing similarly to corrosion risks. Bridges, being exposed to various weather elements and heavy loads, benefit significantly from the use of corrosion inhibitors to prolong their lifespan and safety.
Examples & Analogies
Consider a seaside house that requires special paint to withstand the salty air and high humidity to prevent rust. Similarly, structures like bridges and parking garages need specific 'protective coats' in the form of corrosion inhibitors to ensure they remain durable and safe over many years.
Key Concepts
-
Corrosion Inhibitor: A chemical additive that prevents the corrosion of steel reinforcement.
-
Protective Film: A barrier formed on steel surfaces to protect against environmental damage.
-
Increased pH: Elevating concrete's pH level to enhance the steel's natural oxide layer, thus preventing corrosion.
Examples & Applications
Common examples of corrosion inhibitors include:
Calcium Nitrite: Known to enhance the corrosion resistance of reinforcing steel. It is widely used in marine structures, bridges, and parking garages where corrosion risk is high.
Sodium Benzoate: This is another inhibitor that improves the overall resistance of concrete to corrosion.
Corrosion inhibitors find extensive applications in structures that are particularly vulnerable to environmental challenges, including marine structures, bridges, and parking garages, where the potential exposure to chlorides and moisture is a concern.
In summary, the utilization of corrosion inhibitors in concrete technology plays a crucial role in enhancing the service life of structures in aggressive environments.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In concrete we blend, inhibitors to send, to help our steel stay strong, and together we build long.
Stories
Imagine a noble knight, strong but in danger from rust dragons. He wears a protective armor—this is like a protective film for steel. And every time it rains, the knight's shield increases its power, keeping him safe.
Memory Tools
P.A.R.: Protecting Armor for Reinforcement.
Acronyms
C.R.I. - Calcium for Reinforcement Inhibition.
Flash Cards
Glossary
- Corrosion Inhibitors
Chemical admixtures that protect embedded steel reinforcement in concrete from corrosion.
- Protective Film
A layer formed on the surface of steel to guard against corrosion.
- pH Level
A scale that measures how acidic or basic a solution is; influencing corrosion resistance in concrete.
Reference links
Supplementary resources to enhance your learning experience.
