Hydrocarbons
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Hydrocarbons are organic compounds made entirely of carbon and hydrogen. Can anyone tell me what makes them significant in chemistry?

They are the simplest organic compounds, right?

Exactly! They are indeed the simplest, serving as the basis of organic chemistry. Can anyone guess some sources of hydrocarbons?

Petroleum and natural gas?

Correct! Those are major sources, along with coal. Remember, hydrocarbons are used in fuels and various other applications. Let’s move on to their types!
Types of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What are the two main types of hydrocarbons we studied?

Saturated and unsaturated hydrocarbons?

Great! Saturated hydrocarbons, or alkanes, have only single bonds, with the general formula CₙH₂ₙ₊₂. Can you provide examples?

Methane and Ethane?

Correct! Now, unsaturated hydrocarbons contain double or triple bonds. What are their general formulas?

Alkenes have one double bond, and their formula is CₙH₂ₙ. Alkynes have one triple bond, CₙH₂ₙ₋₂.

Perfect! This foundation will help you understand their behavior in chemical reactions next.
Nomenclature of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Nomenclature is crucial in chemistry. What do you think is important for naming hydrocarbons?

The number of carbon atoms and the type of bond?

Absolutely! The root word tells us the number of carbon atoms, like 'Meth' for 1, 'Eth' for 2, etc. What suffix does 'alkane' use?

-ane for alkanes.

Correct! In contrast, alkenes use -ene, and alkynes use -yne. Let's name a few examples together!
Properties of Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What are some physical properties of hydrocarbons?

They are typically colorless and odorless and usually insoluble in water.

That’s right! How about their chemical properties, especially related to combustion?

They burn in oxygen to produce CO₂ and H₂O.

Exactly! This reaction is crucial for energy production. Also, keep in mind the emission of CO during incomplete combustion.
Uses and Environmental Impact
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone give examples of how we use hydrocarbons in daily life?

As fuels and in making plastics?

Correct! However, we also need to consider the environmental impacts. What are some concerns?

Air pollution from burning fossil fuels and oil spills harming oceans.

Exactly right! It's critical to weigh the benefits against the environmental damage.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the nature of hydrocarbons, including their types (saturated and unsaturated), nomenclature, properties, uses, and environmental impacts. It emphasizes the significance of hydrocarbons in everyday life and their role in organic chemistry.
Detailed
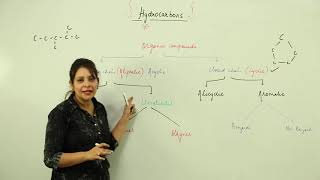
Hydrocarbons
Hydrocarbons are fundamental organic compounds made solely of carbon (C) and hydrogen (H) atoms, forming the backbone of organic chemistry. The sources of hydrocarbons include petroleum, natural gas, and coal. This section categorizes hydrocarbons into two primary types: saturated hydrocarbons (alkanes), which contain only single bonds, and unsaturated hydrocarbons, which include alkenes and alkynes characterized by double and triple bonds, respectively.
Types of Hydrocarbons
- Saturated Hydrocarbons (Alkanes): General formula CₙH₂ₙ₊₂ (e.g., Methane (CH₄), Ethane (C₂H₆))
- Unsaturated Hydrocarbons: Consisting of alkenes (one double bond, CₙH₂ₙ) and alkynes (one triple bond, CₙH₂ₙ₋₂, e.g., Ethene (C₂H₄), Ethyne (C₂H₂)).
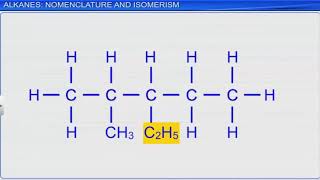
Nomenclature
The naming follows a systematic approach based on the carbon count and the type of bond: prefixes (Meth-, Eth-, Prop-, But-) indicate the number of carbons, while suffixes (-ane, -ene, -yne) indicate the bond types.
Properties
Physical:
- Colorless and odorless (except some gases).
- Insoluble in water but soluble in organic solvents.
- Boiling and melting points increase with molecular chain length.
Chemical:
- Combustion reactions produce CO₂ and H₂O.
- Substitution reactions in alkanes replace hydrogen with halogens, and addition reactions in alkenes/alkynes involve breaking double/triple bonds.
Uses
Hydrocarbons have diverse applications, including fuels (petrol, diesel, LPG), industrial raw materials (plastic, alcohols), and specific uses such as acetylene in welding.
Environmental Impact
Burning fossil fuels results in air pollution and contributes to global warming, while incomplete combustion produces carbon monoxide, a toxic substance, and oil spills cause severe damage to marine life.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Hydrocarbons
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Hydrocarbons are organic compounds made up of only carbon (C) and hydrogen (H) atoms.
● They are the simplest organic compounds and serve as the foundation of organic chemistry.
● Major sources: Petroleum, natural gas, and coal.
Detailed Explanation
Hydrocarbons are a type of organic compound that exclusively contains two elements: carbon (C) and hydrogen (H). This simplicity is what makes them the basic building blocks of organic chemistry. They play a crucial role in the chemical industry and are primarily sourced from petroleum, natural gas, and coal, which are fossil fuel sources found in the Earth's crust.
Examples & Analogies
Think of hydrocarbons as the ' LEGO bricks' of organic chemistry. Just as LEGO bricks can be combined in various ways to build complex structures, hydrocarbons can be joined in a variety of configurations to create diverse organic compounds.
Types of Hydrocarbons
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
13.2 Types of Hydrocarbons
(a) Saturated Hydrocarbons (Alkanes)
● Contain only single bonds between carbon atoms.
● General formula: CₙH₂ₙ₊₂
● Example:
○ Methane (CH₄),
○ Ethane (C₂H₆),
○ Propane (C₃H₈)
(b) Unsaturated Hydrocarbons
● Contain one or more double or triple bonds.
Type Bond Type General Formula Example
Alkenes One double CₙH₂ₙ Ethene (C₂H₄)
bond
Alkynes One triple bond CₙH₂ₙ₋₂ Ethyne (C₂H₂)
Detailed Explanation
Hydrocarbons are categorized into two primary types: saturated and unsaturated. Saturated hydrocarbons, also known as alkanes, only consist of single bonds between carbon atoms, following the general formula CₙH₂ₙ₊₂. Examples include methane, ethane, and propane. In contrast, unsaturated hydrocarbons contain one or more double or triple bonds. Alkenes, which have one double bond, follow the formula CₙH₂ₙ, while alkynes, which have one triple bond, use the formula CₙH₂ₙ₋₂.
Examples & Analogies
Imagine saturated hydrocarbons as solid chains that are tightly packed together, like a single file line at a concert, where everyone holds hands (single bonds) without any gaps. Unsaturated hydrocarbons, however, can be thought of as a flexible group of people who can change positions and form new arrangements, thanks to their double or triple bonds allowing them to bond in different ways.
Nomenclature of Hydrocarbons
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
13.3 Nomenclature of Hydrocarbons
● Based on the number of carbon atoms and bond type.
● Root word indicates number of carbon atoms:
○ Meth– (1), Eth– (2), Prop– (3), But– (4), etc.
● Suffix indicates bond type:
○ –ane (alkane), –ene (alkene), –yne (alkyne)
● Example:
○ C₂H₆ → Ethane
○ C₂H₄ → Ethene
○ C₂H₂ → Ethyne
Detailed Explanation
The names of hydrocarbons are determined by their carbon count and the types of bonds they contain. The root of the name denotes the number of carbon atoms; for instance, 'meth-' indicates 1 carbon, 'eth-' indicates 2, and so on. The suffix of the name describes the bond type – ‘-ane’ for single bonds, ‘-ene’ for double bonds, and ‘-yne’ for triple bonds. For example, the compound C₂H₆ is named ethane because it has two carbon atoms connected by single bonds.
Examples & Analogies
Think of naming hydrocarbons like naming rooms in a hotel. The 'meth,' 'eth,' and 'prop' parts are like room numbers that tell you how large the room is (number of carbons), while ‘-ane,’ ‘-ene,’ and ‘-yne’ describe whether the room has one door (single bond), two doors (double bond), or three doors (triple bond) based on how it connects to other rooms.
Properties of Hydrocarbons
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
13.4 Properties of Hydrocarbons
Physical Properties
● Most are colorless and odorless (except some gases like ethyne).
● Insoluble in water, but soluble in organic solvents.
● Boiling and melting points increase with chain length.
Chemical Properties
● Combustion: Burn in oxygen to produce CO₂ and H₂O.
CH₄ + 2O₂ → CO₂ + 2H₂O + heat
● Substitution reactions (in alkanes): Hydrogen is replaced by halogens in the presence of sunlight.
● Addition reactions (in alkenes and alkynes): Double/triple bonds break to add new atoms.
Detailed Explanation
Hydrocarbons possess distinct physical and chemical properties. Physically, they are generally colorless and odorless, are insoluble in water (but soluble in organic solvents), and their boiling and melting points increase when their carbon chain lengthens. Chemically, they combust in the presence of oxygen, releasing energy and producing carbon dioxide and water. Alkanes can undergo substitution reactions where hydrogen atoms are replaced by halogens, while alkenes and alkynes can participate in addition reactions where new atoms join the molecule by breaking their double or triple bonds.
Examples & Analogies
Consider hydrocarbons like various types of jellybeans. Just as jellybeans come in different colors, shapes, and flavors (properties), hydrocarbons have their unique characteristics as well. When jellybeans are heated (burned), they melt and produce sweet smells (flavors), similar to how hydrocarbons release energy and produce gases when burned. Similarly, just like you can swap a jellybean flavor for another in a jar (substitution), hydrocarbons can replace hydrogen with halogens during chemical reactions.
Uses of Hydrocarbons
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
13.5 Uses of Hydrocarbons
● Fuels: Petrol, diesel, LPG, natural gas (methane).
● Industrial raw materials: Used to manufacture plastics, alcohols, synthetic fibers.
● Welding: Acetylene (C₂H₂) used in oxy-acetylene welding.
Detailed Explanation
Hydrocarbons are incredibly versatile and have numerous applications. They are primarily used as fuels such as petrol, diesel, liquefied petroleum gas (LPG), and natural gas, which power vehicles and heat homes. Additionally, hydrocarbons serve as essential raw materials in industries to produce synthetic products like plastics, alcohols, and fibers. Acetylene, a type of hydrocarbon, is notably used in welding processes due to its high flame temperature.
Examples & Analogies
Imagine hydrocarbons as the essential ingredients in a chef’s kitchen. Just as a good chef uses various ingredients to create different dishes (fuels for energy), they also use the same ingredients to bake, fry, and fabricate unique recipes (industrial materials for products). For instance, natural gas might be likened to cooking oil, powering a stove, while acetylene is like a blowtorch, perfect for welding things together.
Environmental Impact of Hydrocarbons
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
13.6 Environmental Impact
● Incomplete combustion produces carbon monoxide (CO) – a toxic gas.
● Burning fossil fuels contributes to air pollution and global warming.
● Hydrocarbons in oil spills pollute water bodies and harm marine life.
Detailed Explanation
While hydrocarbons are valuable, their combustion can have negative environmental consequences. When hydrocarbons burn incompletely, they can produce carbon monoxide, a toxic gas that is harmful to health. The burning of fossil fuels contributes significantly to air pollution and is a major factor in global warming due to greenhouse gas emissions. Furthermore, accidents like oil spills can lead to hydrocarbons contaminating water bodies, posing serious threats to aquatic life and ecosystems.
Examples & Analogies
Think of hydrocarbons as a car running down a street. If the car exhaust is like emissions from burning hydrocarbons, incomplete combustion is akin to the engine choking and releasing harmful fumes – dangerous for pedestrians (air pollution). An oil spill can be imagined as the car spilling oil on the road, creating a slippery and hazardous surface for wildlife (marine life).
Key Concepts
-
Hydrocarbons consist only of carbon and hydrogen, forming the basis of organic chemistry.
-
They are categorized into saturated (alkanes) and unsaturated (alkenes and alkynes) based on their bonding structure.
-
Naming conventions involve prefixes for carbon count and suffixes for bond types.
-
Physical properties generally include being colorless and insoluble in water; chemical properties include combustion and reaction types.
-
Hydrocarbons have various uses but also significant environmental concerns.
Examples & Applications
Methane (CH₄) is the simplest alkane and is used widely as a fuel.
Ethyne (C₂H₂), an alkyne, is commonly used in welding due to its high flame temperature.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrocarbons in a chain, single or double, we explain!
Stories
Imagine a world where every fuel and plastic comes from simple carbon-hydrogen links that help keep our vehicles going and homes warm.
Memory Tools
To remember hydrocarbons: H for Hydrogen, C for Carbon – H comes first in life!
Acronyms
H.A.C – Hydrocarbons, Alkanes, Chains illustrate the basics!
Flash Cards
Glossary
- Hydrocarbon
An organic compound made only of carbon and hydrogen atoms.
- Alkane
A saturated hydrocarbon with only single bonds, general formula CₙH₂ₙ₊₂.
- Alkene
An unsaturated hydrocarbon containing one double bond, general formula CₙH₂ₙ.
- Alkyne
An unsaturated hydrocarbon with one triple bond, general formula CₙH₂ₙ₋₂.
- Combustion
A chemical reaction that occurs when hydrocarbons react with oxygen, producing energy, CO₂, and H₂O.
- Nomenclature
A systematic naming process for chemical compounds.
Reference links
Supplementary resources to enhance your learning experience.
