Types of Hydrocarbons
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore hydrocarbons, specifically focusing on the two main types: saturated and unsaturated. Can anyone tell me what a hydrocarbon is?

A hydrocarbon is an organic compound made up of carbon and hydrogen.

That's right! Now, saturated hydrocarbons are known as alkanes. Does anyone remember the general formula for alkanes?

I think it's CₙH₂ₙ₊₂?

Excellent! This indicates that for every carbon atom, we can find two hydrogen atoms plus two more. Can you name a few examples of alkanes?

Methane and propane!

Great examples! To remember them, think of the acronym 'MEP': Methane, Ethane, Propane. This covers our first three alkanes. Now, let’s move on to unsaturated hydrocarbons.
Unsaturated Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Unsaturated hydrocarbons include alkenes and alkynes. Can anyone tell me what distinguishes them from alkanes?

They have either double or triple bonds?

Exactly! Alkenes contain one or more double bonds with the formula CₙH₂ₙ. Can someone give me an example?

Ethene is an example of an alkene!

Correct! Now, what about alkynes? Who can define them?

Alkynes have triple bonds and the formula CₙH₂ₙ₋₂, right?

Exactly! A well-known example is ethyne. Let's summarize: Alkenes have double bonds, and alkynes have triple bonds, allowing them to form additional bonds with hydrogen. This property influences their reactivity!
Reactivity and Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We have now covered the types of hydrocarbons, but why is it important to differentiate between them? What do you think?

Maybe because they react differently with other compounds?

Absolutely! Saturated hydrocarbons like alkanes are generally less reactive than unsaturated ones. Their structure leads to different applications as well, like fuels or in molding plastics. Can anyone think of an example where we use ethene?

It can be used to create plastics, right?

Exactly! Ethene is crucial in polymer production. So, in summary, understanding the differences helps us utilize these hydrocarbons effectively across various industries.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hydrocarbons are divided into saturated and unsaturated types. Saturated hydrocarbons, or alkanes, have only single bonds and are represented by the general formula CₙH₂ₙ₊₂. Unsaturated hydrocarbons include alkenes, with one or more double bonds (CₙH₂ₙ), and alkynes, with one or more triple bonds (CₙH₂ₙ₋₂), with specific examples provided for each type.
Detailed
Detailed Summary of Types of Hydrocarbons
In this section, we examine the types of hydrocarbons, which are organic compounds made exclusively of carbon (C) and hydrogen (H) atoms. Hydrocarbons are primarily categorized into:
1. Saturated Hydrocarbons (Alkanes)
- Definition: Alkanes have only single bonds between carbon atoms. This characteristic makes them saturated with hydrogen atoms, meaning they cannot bond with any more hydrogens.
- General Formula: The structural representation follows the formula CₙH₂ₙ₊₂, where n represents the number of carbon atoms.
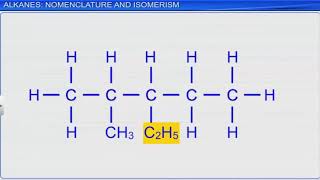
- Examples: Common examples include Methane (CH₄), Ethane (C₂H₆), and Propane (C₃H₈).
2. Unsaturated Hydrocarbons**
- Definition: These hydrocarbons contain one or more double or triple bonds, making them unsaturated as they can accommodate additional hydrogen atoms.
- Types:
- Alkenes: Hydrocarbons with one double bond, following the formula CₙH₂ₙ. An example is Ethene (C₂H₄).
- Alkynes: Hydrocarbons with at least one triple bond, represented by the formula CₙH₂ₙ₋₂. An example is Ethyne (C₂H₂).
Understanding these categories is crucial as they form the foundation for further studies in organic chemistry, including functional groups, reactivity, and applications of these compounds.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Saturated Hydrocarbons (Alkanes)
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Saturated Hydrocarbons (Alkanes)
● Contain only single bonds between carbon atoms.
● General formula: CₙH₂ₙ₊₂
● Example:
○ Methane (CH₄),
○ Ethane (C₂H₆),
○ Propane (C₃H₈)
Detailed Explanation
Saturated hydrocarbons, also known as alkanes, are a class of hydrocarbons that only contain single bonds between carbon atoms. This means the carbon atoms are connected in a way that allows for the maximum number of hydrogen atoms to bond with them. The general formula for alkanes is CₙH₂ₙ₊₂, where 'n' represents the number of carbon atoms. For example, if 'n' is 1, the hydrocarbon is methane (CH₄); when 'n' is 2, it's ethane (C₂H₆); and when 'n' is 3, it's propane (C₃H₈). This structure influences their chemical properties and makes them a fundamental class of hydrocarbons in organic chemistry.
Examples & Analogies
Think of saturated hydrocarbons like a family of balloons filled to their limit. Each carbon atom (the balloon) can only connect to other carbons and hydrogens in a way that allows them to stay fully inflated (saturated with hydrogen). For instance, propane can be likened to three balloons tied together, allowing the maximum number of hydrogen 'strings' to connect, representing how they bond to form a larger molecule.
Unsaturated Hydrocarbons
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Unsaturated Hydrocarbons
● Contain one or more double or triple bonds.
Type Bond Type General Formula Example
Alkenes One double bond CₙH₂ₙ General Formula: CₙH₂ₙ
E.g., Ethene (C₂H₄)
Alkynes One triple bond CₙH₂ₙ₋₂ General Formula: CₙH₂ₙ₋₂
E.g., Ethyne (C₂H₂)
Detailed Explanation
Unsaturated hydrocarbons are compounds that contain one or more double or triple bonds between carbon atoms, leading to fewer hydrogen atoms attached. This means these hydrocarbons are not fully 'saturated' with hydrogen like alkanes. Alkenes, which contain one double bond, have a general formula of CₙH₂ₙ. For example, ethene (C₂H₄) is an alkene with two carbon atoms. Alkynes have one triple bond, represented by the general formula CₙH₂ₙ₋₂; an example is ethyne (C₂H₂). The presence of these multiple bonds allows for different chemical reactions and properties compared to saturated hydrocarbons.
Examples & Analogies
You can think of unsaturated hydrocarbons like a friendship circle where not everyone is holding hands (double or triple bonds). In the case of alkenes, imagine a situation where two friends (carbons) clasp hands tightly (double bond) but still have room for more friends to join (bonds available for reactions). Conversely, in alkynes, it's like a super tight grip (triple bond) that makes it harder for new friends (hydrogens) to join, leaving fewer bonding spots available.
Key Concepts
-
Saturated Hydrocarbons: Contain only single bonds and follow the formula CₙH₂ₙ₊₂.
-
Unsaturated Hydrocarbons: Contain double or triple bonds, allowing for more hydrogen atoms to bond.
-
Alkenes: A subset of unsaturated hydrocarbons with one double bond.
-
Alkynes: A subset of unsaturated hydrocarbons with one triple bond.
Examples & Applications
Methane (CH₄) is a saturated hydrocarbon (alkane) showing the simplest structure.
Ethene (C₂H₄) is an unsaturated hydrocarbon (alkene) with one double bond.
Ethyne (C₂H₂) is an unsaturated hydrocarbon (alkyne) exhibiting a triple bond.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Alkanes, they are neat, with a bond that's sweet. Alkenes make it twist, with double on the list.
Stories
In a world of hydrocarbons lived Al and his twin, Ole. Al had single connections, while Ole loved to tie up with double bonds.
Memory Tools
A for Alkane, double the A for Alkenes, triple the A for Alkynes.
Acronyms
HARD
for Hydrocarbon
for Alkane
for Reactivity
for Double bonds.
Flash Cards
Glossary
- Hydrocarbon
An organic compound consisting entirely of carbon and hydrogen.
- Saturated Hydrocarbons
Hydrocarbons that only contain single bonds, allowing for maximum hydrogen saturation.
- Alkanes
A type of saturated hydrocarbon with the formula CₙH₂ₙ₊₂.
- Unsaturated Hydrocarbons
Hydrocarbons that contain one or more double or triple bonds.
- Alkenes
Hydrocarbons with at least one double bond, represented by the formula CₙH₂ₙ.
- Alkynes
Hydrocarbons with at least one triple bond, represented by the formula CₙH₂ₙ₋₂.
Reference links
Supplementary resources to enhance your learning experience.
