Saturated Hydrocarbons (Alkanes)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Saturated Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today, we're diving into saturated hydrocarbons, also known as alkanes. These are special because they only have single bonds between carbon atoms. Can anyone tell me what a hydrocarbon is?

Are hydrocarbons just made of carbon and hydrogen?

That's right, Student_1! Hydrocarbons consist solely of carbon and hydrogen atoms. Can anyone provide an example of a saturated hydrocarbon?

Like methane?

Exactly! Methane is C₁H₄, the simplest saturated hydrocarbon. Remember, the general formula for alkanes is CnH2n+2.
Understanding the General Formula
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into the general formula of alkanes. For n carbon atoms, what's the formula?

It's CnH2n+2!

Correct! So if we have two carbons, what is the formula?

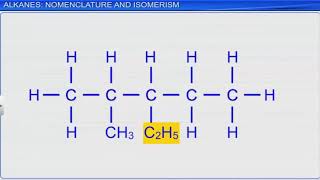
That will be C₂H₆, which is ethane!

And if we have three carbons, that's propane, C₃H₈!

Great job, everyone! You're really grasping this concept well. Remember, knowing the formula is key for understanding more complex hydrocarbons.
Characteristics and Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So why are alkanes significant? Let’s talk about their properties. Who can describe one characteristic of alkanes?

They don't have double or triple bonds!

Correct! This single-bonded structure also makes them more stable compared to unsaturated hydrocarbons. Any other properties?

They are colorless and odorless?

Exactly! Most alkanes are colorless and odorless, especially at room temperature. Let's recap: Name an example and its formula.

Ethane, which is C₂H₆.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
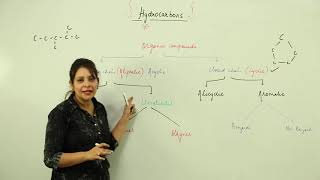
Alkanes are a type of hydrocarbon with the general formula CnH2n+2, indicating that they only contain single bonds between carbon atoms. Common examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈). These compounds are essential in organic chemistry and are found in various natural resources.
Detailed
Saturated Hydrocarbons (Alkanes)
Saturated hydrocarbons, commonly referred to as alkanes, are organic compounds consisting exclusively of carbon (C) and hydrogen (H) atoms connected predominantly by single bonds. Their general molecular formula is represented as CnH2n+2, which underscores their saturated nature, meaning each carbon atom is bonded to the maximum number of hydrogen atoms possible without forming double or triple bonds.
Key Characteristics:
- Single Bonds Only: Alkanes can be differentiated from unsaturated hydrocarbons due to their single bond structures, which impacts their chemical behavior.
- Examples: Notable members of the alkane family include:
- Methane (CH₄): The simplest alkane, consisting of one carbon atom.
- Ethane (C₂H₆): Comprises two carbon atoms.
- Propane (C₃H₈): Contains three carbon atoms.
This segment of hydrocarbons establishes a foundational understanding necessary for delving deeper into organic chemistry topics, such as reactions, nomenclature, and properties.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Saturated Hydrocarbons
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Contain only single bonds between carbon atoms.
Detailed Explanation
Saturated hydrocarbons, also known as alkanes, are organic compounds that consist solely of carbon and hydrogen atoms. The defining characteristic of these compounds is that the carbon atoms are linked exclusively by single bonds. This stability and structure allow them to be fully 'saturated' with hydrogen atoms, meaning they contain the maximum number of hydrogen atoms attached to the carbon skeleton.
Examples & Analogies
You can think of saturated hydrocarbons as a stable chain where each carbon atom is holding hands with hydrogen atoms. Just like how a group of friends can only hold hands with one person on each side (making it a simple, stable chain), saturated hydrocarbons can only link each carbon to hydrogen with single bonds.
General Formula of Alkanes
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● General formula: CₙH₂ₙ₊₂
Detailed Explanation
The general formula for saturated hydrocarbons, or alkanes, is given by CₙH₂ₙ₊₂, where 'n' represents the number of carbon atoms in the molecule. For every carbon atom, there are twice as many hydrogen atoms plus an additional two hydrogen atoms. This formula reflects how carbon and hydrogen atoms combine within saturated hydrocarbons.
Examples & Analogies
Imagine each carbon is like a seat in a car. For every seat (carbon atom), you need a specific number of additional passengers (hydrogen atoms) to fill the car correctly. The formula CₙH₂ₙ₊₂ shows how to calculate how many people can get in based on how many seats you have.
Examples of Saturated Hydrocarbons
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Example:
○ Methane (CH₄),
○ Ethane (C₂H₆),
○ Propane (C₃H₈)
Detailed Explanation
Saturated hydrocarbons include various simple compounds. Methane (CH₄) is the simplest alkane, composed of one carbon atom and four hydrogen atoms. Ethane (C₂H₆) consists of two carbon atoms linked together, surrounded by six hydrogen atoms. Propane (C₃H₈) features three carbon atoms with eight hydrogen atoms. These examples illustrate how the number of carbon atoms directly affects the composition of saturated hydrocarbons.
Examples & Analogies
Think of methane as a single room filled with four balloons (hydrogen atoms). If you add another room (a second carbon) for ethane, you can fit more balloons, totaling six. When you bring in a third room for propane, now you can fit even more balloons – eight in total! Each carbon 'room' allows for more hydrogen 'balloons' to be accommodated.
Key Concepts
-
Saturated Hydrocarbons: Organic compounds with only single bonds.
-
General Formula of Alkanes: CnH2n+2, indicating the relationship between carbon and hydrogen.
-
Examples of Alkanes: Methane, Ethane, and Propane.
Examples & Applications
Methane (CH₄): The simplest alkane with one carbon atom.
Ethane (C₂H₆): An alkane with two carbon atoms.
Propane (C₃H₈): An alkane consisting of three carbon atoms.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Alkanes are stable as can be, single bonds are their decree.
Stories
In a kingdom of carbon, each carbon wishes to hold hands only with hydrogen, forming stable pairs and living harmoniously with no double trouble.
Memory Tools
C for Carbon, H for Hydrogens, together they stand, making alkanes so grand!
Acronyms
SHINE
Saturated Hydrocarbons Include Only Non-Double bonds.
Flash Cards
Glossary
- Saturated Hydrocarbon
An organic compound containing only single bonds between carbon atoms.
- Alkane
A type of saturated hydrocarbon with the general formula CnH2n+2.
- General Formula
A formula that shows the relationship between the number of carbon atoms and hydrogen atoms in a hydrocarbon.
Reference links
Supplementary resources to enhance your learning experience.
