Physical Properties
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hydrocarbon Physical Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start our discussion on the physical properties of hydrocarbons. Can anyone tell me some properties of hydrocarbons?

I know they are colorless and mostly odorless.

Exactly! Most hydrocarbons are indeed colorless and odorless, except for some like ethyne. This means they are often hard to detect. Why do you think this is important?

Maybe because it makes them easier to handle in larger quantities without safety concerns?

Yes, great insight! It can also contribute to safety in handling, as it reduces immediate chemical detection. Now, who remembers if hydrocarbons can dissolve in water?

They don't dissolve in water, they’re insoluble.

Correct! This insolubility is a defining characteristic due to their nonpolar nature. They do, however, dissolve in organic solvents. Let's recap: colorless, mostly odorless, and insoluble in water but soluble in organic solvents. Any questions about these properties?
Solubility and Miscibility
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've covered solubility, let’s dive deeper. Who can tell me why hydrocarbons are insoluble in water?

Because they are nonpolar and water is polar, right?

Exactly, nonpolar substances do not mix with polar ones. This concept is fundamental in chemistry. Can someone provide an example of an organic solvent?

Maybe something like ethanol or hexane?

Yes, both of those can dissolve hydrocarbons while being organic solvents themselves! Now, let’s discuss boiling and melting points. Do they increase with the length of the carbon chain?

Yes, longer chains have higher boiling and melting points.

Correct! This increase is due to the enhanced van der Waals forces. A longer chain means more surface area for these forces to act upon. Well done on these discussions, guys!
Chain Length Impact
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore how the length of a hydrocarbon chain affects its physical properties. Can anyone summarize how chain length relates to boiling points?

As the chain gets longer, the boiling and melting points increase!

Exactly! This is an essential concept because it helps us predict the behavior of different hydrocarbons. What might be a practical implication of knowing this?

It can affect how we use them as fuels, right? Longer chains might be better for different applications!

Absolutely! Additionally, this property is crucial in industrial processes. Understanding these differences helps chemists select the right hydrocarbon for specific uses. Great connections, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the physical properties of hydrocarbons, emphasizing that most are colorless and odorless, insoluble in water, and have boiling and melting points that increase with chain length. These properties are foundational for understanding their behavior in different environments.
Detailed
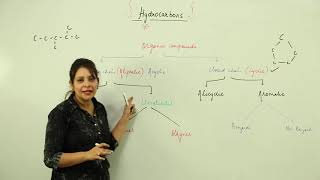
Physical Properties of Hydrocarbons
Hydrocarbons, composed solely of carbon and hydrogen atoms, exhibit several significant physical properties that distinguish them from other organic compounds. Most hydrocarbons are colorless and odorless, making them generally unrecognizable in gaseous forms unless specifically identified. A key characteristic is their insolubility in water; hydrocarbons do not mix well with water due to their nonpolar nature, which diverges from the polar characteristics of water. However, they are soluble in organic solvents, which often facilitates their extraction and application in various industrial processes. Additionally, the boiling and melting points of hydrocarbons typically increase with chain length. This phenomenon can be attributed to an increase in molecular weight and surface area, which enhances van der Waals forces among molecules. Understanding these physical properties is crucial for students and professionals in chemistry and related fields as they establish the groundwork for more complex interactions and reactions involving hydrocarbons.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
General Characteristics of Hydrocarbons
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Most are colorless and odorless (except some gases like ethyne).
Detailed Explanation
Hydrocarbons are mainly characterized by being colorless and odorless. This means that when you look at most hydrocarbons, they do not have any color and do not emit any smell. This characteristic holds true for the majority of hydrocarbons, making them less detectable in their pure forms. However, there are exceptions, such as ethyne, which can have a distinct smell due to its unique structure.
Examples & Analogies
Think of hydrocarbons like water—when water is pure, it looks clear and has no smell, making it hard to notice. Just like we can find some substances that are odorless but have unique uses, hydrocarbons like ethyne have different characteristics that can be identified when we refer to their specific uses in industry.
Solubility in Water and Organic Solvents
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Insoluble in water, but soluble in organic solvents.
Detailed Explanation
Hydrocarbons generally do not dissolve in water, which means if you were to mix them with water, they would float or settle rather than blend. This property is due to the molecular structure of hydrocarbons, which makes them non-polar. In contrast, they can dissolve in organic solvents, which are also typically non-polar. This characteristic is important for various applications in chemistry and industry, as it influences how hydrocarbons interact with other substances.
Examples & Analogies
Imagine trying to mix oil and water. Just like oil, hydrocarbons do not mix well with water. However, if you mix oil with another fat or oil-based substance (like salad dressing), they blend perfectly. This is similar to how hydrocarbons behave in organic solvents, showing us how different substances interact based on their chemical properties.
Effects of Chain Length on Boiling and Melting Points
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Boiling and melting points increase with chain length.
Detailed Explanation
The boiling and melting points of hydrocarbons are influenced by the length of their carbon chains. As the chain length increases (for example, going from a small molecule like methane to larger molecules like octane), the boiling and melting points also increase. This is due to the greater number of bonds and interactions between larger molecules, which require more energy to break apart, hence the higher temperatures needed for boiling or melting.
Examples & Analogies
Consider a long train versus a short train. A long train has many cars and is a lot heavier than a short train. It takes more effort (energy) to get the long train moving or to stop it than the short train. Similarly, longer carbon chains in hydrocarbons are heavier and require more energy (higher temperatures) to change their states from solid to liquid (melting) or liquid to gas (boiling).
Key Concepts
-
Colorless and Odorless: Most hydrocarbons are colorless and odorless, which makes detection difficult.
-
Insoluble in Water: Hydrocarbons do not dissolve in water due to their nonpolar nature.
-
Soluble in Organic Solvents: They can dissolve in organic solvents, facilitating various industrial applications.
-
Chain Length Effect: The boiling and melting points of hydrocarbons increase with the length of their carbon chains.
Examples & Applications
Methane (CH₄), the simplest hydrocarbon, is colorless and odorless and is used as a fuel.
Octane (C₈H₁₈) has a higher boiling point than butane (C₄H₁₀) due to its longer chain length.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrocarbons flow and often glow, colorless and odorless, this you should know.
Stories
Imagine a rainbow of hydrocarbons, where each color represents a different chain length. The longer the chain, the higher they soar, just like their boiling points, bringing fuels to the fore.
Memory Tools
C-O-W: Colorless, Odorless, Water-insoluble. Remember the C-O-W concept for hydrocarbons!
Acronyms
HIPS
Hydrocarbons are Insoluble in Polar Solvents.
Flash Cards
Glossary
- Hydrocarbon
An organic compound consisting exclusively of hydrogen and carbon atoms.
- Insoluble
Incapable of being dissolved in a solvent, such as water.
- Organic Solvent
A solvent that contains carbon and can dissolve hydrocarbons.
- Boiling Point
The temperature at which a substance transitions from a liquid to a gas.
- Melting Point
The temperature at which a solid transitions to a liquid.
- Van der Waals Forces
Weak attractions between molecules or parts of molecules that contribute to the physical properties of substances.
Reference links
Supplementary resources to enhance your learning experience.
