Unsaturated Hydrocarbons
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Unsaturated Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into unsaturated hydrocarbons! Can anyone tell me what makes a hydrocarbon 'unsaturated'?

Is it because they have double or triple bonds?

That's correct, Student_1! Unsaturated hydrocarbons contain one or more double or triple bonds, unlike saturated hydrocarbons that only have single bonds. Can anyone name the two main types of unsaturated hydrocarbons?

Alkenes and alkynes!

Exactly! Alkenes have double bonds, like Ethene, and alkynes have triple bonds, like Ethyne. Let's remember Alkenes have 'ene' and Alkynes have 'yne' in their names. Great mnemonic!

So, what are the formulas for these types?

Great question! For alkenes, the general formula is CₙH₂ₙ, and for alkynes, it's CₙH₂ₙ₋₂. This means the amount of hydrogen decreases by 2 for each triple bond.

Why does that happen?

Since the triple bond takes up more bonding capacity, there's less space for hydrogen. Does everyone understand?

Yes!
Reactivity of Unsaturated Hydrocarbons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know about the structure, let's talk about how unsaturated hydrocarbons react. What can you tell me about the reactivity of alkenes and alkynes?

They’re more reactive than alkanes because of the double and triple bonds.

Exactly! The double bonds in alkenes can undergo addition reactions, where new atoms can be added to the carbon chain. For example, an alkene reacting with hydrogen can form an alkane.

What about alkynes?

Good question! Alkynes also participate in addition reactions and are typically even more reactive than alkenes due to the presence of a triple bond.

Can we perform these reactions in the lab?

Yes, many of these reactions are performed in laboratory settings, especially for making useful compounds. It's important to use proper safety measures since these reactions can be vigorous.

So, understanding these properties is really useful for real-world applications?

Absolutely! Understanding these compounds allows us to synthesize materials such as plastics and fuels.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section covers unsaturated hydrocarbons, including their structure, general formulas, and examples of alkenes and alkynes. Understanding these hydrocarbons is essential in organic chemistry, as they play crucial roles in various chemical reactions.
Detailed
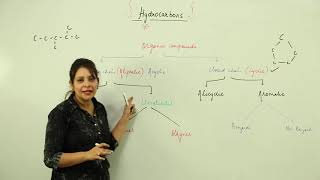
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are organic compounds that contain one or more double or triple bonds between carbon atoms. Unlike saturated hydrocarbons (alkanes) that consist solely of single bonds, the presence of these multiple bonds introduces unique properties and reactivities. The two main categories of unsaturated hydrocarbons are alkenes and alkynes.
Key Points:
- Structure and Bonding: Unsaturated hydrocarbons can be classified into two types based on their bond types:
- Alkenes: Contain one double bond. A common example is Ethene (C₂H₄), with the general formula CₙH₂ₙ.
- Alkynes: Feature one triple bond, with examples such as Ethyne (C₂H₂) and follow the general formula CₙH₂ₙ₋₂.
- General Formulas: The general formulas for these hydrocarbons are crucial for understanding their composition:
- Alkenes: CₙH₂ₙ
- Alkynes: CₙH₂ₙ₋₂
- Reactivities: The double or triple bonds introduce unique chemical behavior, such as addition reactions in alkenes and alkynes, making them more reactive than their saturated counterparts.
Understanding these family of hydrocarbons is fundamental in organic chemistry, as it sets the stage for the study of their reactions and their applications in various fields, including industry and environmental science.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Unsaturated Hydrocarbons
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Contain one or more double or triple bonds.
Detailed Explanation
Unsaturated hydrocarbons are types of hydrocarbons that include one or more double bonds or triple bonds between carbon atoms. This characteristic differentiates them from saturated hydrocarbons, which only feature single bonds between carbon atoms. The presence of these multiple bonds gives unsaturated hydrocarbons unique chemical properties.
Examples & Analogies
Think of unsaturated hydrocarbons like a tightly packed group of friends standing close together (single bonds). When one friend (a bond) decides to hold two others’ hands (double bonds), there's not only more connection but also a different feel to the gathering. This added connection impacts how they interact with new friends (other elements).
Types of Unsaturated Hydrocarbons
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Type Bond Type General Formula Example
Alkenes One double bond CₙH₂ₙ Ethene (C₂H₄)
Alkynes One triple bond CₙH₂ₙ₋₂ Ethyne (C₂H₂)
Detailed Explanation
There are two main types of unsaturated hydrocarbons: alkenes and alkynes. Alkenes contain at least one double bond between carbon atoms, and their general formula is CₙH₂ₙ. An example of an alkene is Ethene (C₂H₄), which has the formula indicating two carbon atoms and four hydrogen atoms. On the other hand, alkynes contain at least one triple bond between carbon atoms, with the general formula CₙH₂ₙ₋₂. An example of an alkyne is Ethyne (C₂H₂), which has two carbon atoms and two hydrogen atoms.
Examples & Analogies
Imagine a family tree with siblings representing carbon atoms. In alkenes, some siblings are holding hands (double bonds), creating an extra link. In alkynes, siblings are not only holding hands but also embracing (triple bonds), making their connection even more intense. Each type of connection (bond) changes how they engage with others (additional atoms).
Key Concepts
-
Unsaturated Hydrocarbons: Contain double or triple bonds, making them more reactive than saturated hydrocarbons.
-
Alkenes: Unsaturated hydrocarbons with one double bond and general formula CₙH₂ₙ.
-
Alkynes: Unsaturated hydrocarbons with one triple bond and general formula CₙH₂ₙ₋₂.
Examples & Applications
Ethene (C₂H₄) is an example of an alkene.
Ethyne (C₂H₂) is an example of an alkyne.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Alkenes have 'ene', with a bond that's double, alkynes have 'yne', making reactions a hubble!
Stories
Once there were two friends, Alkenes and Alkynes. Alkenes were always double busy making connections, while Alkynes had triple the fun with their stronger bonds!
Memory Tools
Remember: 'A' for Alkyne and 'A' for a triple bond, 'E' for Alkene and 'E' for a double bond.
Acronyms
Use 'DAC' for 'Double = Alkene', 'Triple = Alkyne' to recall the types.
Flash Cards
Glossary
- Unsaturated Hydrocarbons
Organic compounds with one or more double or triple bonds between carbon atoms.
- Alkenes
Unsaturated hydrocarbons containing at least one double bond; general formula is CₙH₂ₙ.
- Alkynes
Unsaturated hydrocarbons containing at least one triple bond; general formula is CₙH₂ₙ₋₂.
Reference links
Supplementary resources to enhance your learning experience.
