Nomenclature of Hydrocarbons
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hydrocarbon Nomenclature
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re going to break down the nomenclature of hydrocarbons. Can anyone tell me what nomenclature means?

Is it how we name things in chemistry?

Exactly! Nomenclature is a system of naming chemical compounds. For hydrocarbons, it’s based on the number of carbon atoms and the types of bonds present. What are the different types of bonds we encounter in hydrocarbons?

Single, double, and triple bonds!

Correct! And those types of bonds influence the naming convention we use. Let’s hear some root words for the number of carbon atoms.

Meth- for one, Eth- for two, and Prop- for three!

Great memory! Remember these root words as they’ll help us understand the more complex names of the hydrocarbons.
Bond Types and Their Suffixes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss the suffixes. The suffix indicates the type of bond in the hydrocarbon. Can someone tell me the suffix for alkanes?

-ane for single bonds.

Exactly! And what about alkenes and alkynes?

-ene for double bonds and -yne for triple bonds.

Perfect! For example, if I have C₂H₆, how would I name it?

That would be ethane since it has a single bond.

Exactly! And C₂H₄?

Ethene because it contains a double bond.

Great! You’re all getting the hang of this. Remember that understanding these suffixes is crucial for accurately identifying hydrocarbon compounds.
Examples and Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s solidify our understanding with some examples. If I say C₂H₂, how would you name that compound?

That’s ethyne because it has a triple bond.

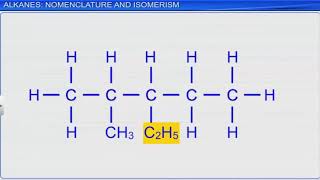
Correct! Now, what about C₄H₁₀?

That’s butane because it has only single bonds.

Excellent! So, how do we know if a compound is saturated or unsaturated based on its name?

Saturated compounds have the -ane suffix, while unsaturated ones have -ene or -yne.

Right again! This distinction is essential when studying physical and chemical properties of hydrocarbons. Let’s keep practicing more examples.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hydrocarbons are named using a systematic method that combines root words indicating the number of carbon atoms with suffixes that indicate the type of bonds—single, double, or triple. For example, C₂H₆ is named ethane, while C₂H₄ is ethene. Understanding this nomenclature is critical for identifying and communicating about hydrocarbons in chemistry.
Detailed
Nomenclature of Hydrocarbons
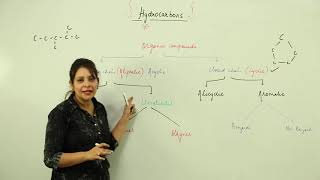
Hydrocarbons are organic compounds composed solely of carbon and hydrogen atoms. Their nomenclature relies on a systematic method rooted in organic chemistry principles. The naming of these compounds is dictated by two main factors: the number of carbon atoms present and the type of bonds between these carbon atoms.
Key Elements of Nomenclature:
- Root Words: The root word signifies the number of carbon atoms:
- Meth- (1 carbon), Eth- (2 carbons), Prop- (3 carbons), But- (4 carbons), etc.
- Suffix: The suffix indicates the type of carbon bonds:
- -ane for saturated hydrocarbons (alkanes with only single bonds),
- -ene for unsaturated hydrocarbons with one double bond (alkenes), and
- -yne for unsaturated hydrocarbons with one triple bond (alkynes).
Examples:
- Ethane (C₂H₆) - saturated with only single bonds.
- Ethene (C₂H₄) - contains a double bond.
- Ethyne (C₂H₂) - contains a triple bond.
Understanding the nomenclature of hydrocarbons is essential for the study of organic chemistry, as it allows chemists to accurately identify and communicate about these fundamental compounds.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Basic Concepts of Nomenclature
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Based on the number of carbon atoms and bond type.
Detailed Explanation
The nomenclature of hydrocarbons involves naming these compounds based on two fundamental aspects. First, the number of carbon atoms in the molecule determines the root word used in the name. Second, the type of bonds between carbon atoms—single, double, or triple bonds—determines the suffix that describes the molecule. For instance, if the molecule contains only single bonds, it will have a suffix of ‘-ane.’
Examples & Analogies
Think of naming a pet based on its breed and color. The breed represents the type, similar to the number of carbon atoms, and the color indicates specific characteristics, analogous to the bond type.
Root Words Indicating Carbon Atoms
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Root word indicates number of carbon atoms:
○ Meth– (1), Eth– (2), Prop– (3), But– (4), etc.
Detailed Explanation
Each root word used in hydrocarbon nomenclature signifies the number of carbon atoms present in the molecule. For example, 'Meth-' is associated with one carbon atom, 'Eth-' signifies two carbon atoms, 'Prop-' indicates three carbon atoms, and 'But-' refers to four carbon atoms. This systematic approach helps in quickly identifying the number of carbons in the compound based solely on its name.
Examples & Analogies
Imagine a family tree where each name indicates the number of family members. 'Meth' could be a single child, 'Eth' could represent two siblings, and so forth, making it easier to understand the family size just by looking at their names.
Suffixes Indicating Bond Types
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Suffix indicates bond type:
○ –ane (alkane), –ene (alkene), –yne (alkyne)
Detailed Explanation
In hydrocarbon nomenclature, the suffix of the compound name reveals the type of bonds present between the carbon atoms. For instance, '-ane' signifies that the compound is an alkane with all single bonds, '-ene' indicates that it is an alkene with at least one double bond, and '-yne' denotes that the compound is an alkyne containing at least one triple bond. This helps chemists quickly ascertain the nature of the chemical bonds just by glancing at the name.
Examples & Analogies
Think of a book title where the title suffix tells you the genre. A book titled 'Drama' means it’s a drama story (similar to '–ane'), while one titled 'Romance' suggests a love story (similar to '–ene'), and a 'Thriller' indicates suspense (like '–yne').
Examples of Hydrocarbon Nomenclature
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Example:
○ C₂H₆ → Ethane
○ C₂H₄ → Ethene
○ C₂H₂ → Ethyne
Detailed Explanation
To better understand nomenclature, we look at specific examples. C₂H₆ is named 'Ethane' because it contains two carbon atoms and is an alkane (single bonds). C₂H₄ is 'Ethene,' which indicates the same two carbon atoms but with at least one double bond. Lastly, C₂H₂ is called 'Ethyne,' denoting the two carbon atoms with a triple bond. Each name is constructed by combining the root indicating the number of carbons with the suffix that indicates the bond type.
Examples & Analogies
Like naming a car model, where the model name gives you hints at its features. 'Model A' with single doors could be likened to 'Ethane,' while 'Model B' with double doors could represent 'Ethene,' and a performance 'Model C' with a turbo feature could represent 'Ethyne.'
Key Concepts
-
Root Words: Indicate the number of carbon atoms (Meth-, Eth-, Prop-, But-).
-
Suffixes: Indicate bond types (-ane for alkanes, -ene for alkenes, -yne for alkynes).
-
Saturated vs. Unsaturated: Saturated hydrocarbons have only single bonds, while unsaturated hydrocarbons have double or triple bonds.
Examples & Applications
Ethane (C₂H₆) - saturated with only single bonds.
Ethene (C₂H₄) - contains a double bond.
Ethyne (C₂H₂) - contains a triple bond.
Understanding the nomenclature of hydrocarbons is essential for the study of organic chemistry, as it allows chemists to accurately identify and communicate about these fundamental compounds.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For single bonds, there's -ane; for double bonds, -ene is the name; for triple bonds, -yne's the game!
Stories
Once upon a time in the land of hydrocarbons, Meth and Eth had a party. Meth was simple, with his single bond, but Eth was a bit fancy with his double bond. Prop showed up with a three-way connection, but But knew he'd always be linked with just one connection, being saturated!
Memory Tools
To remember the bond types: 'Aunt Anne's Ever-Young' - -ane for single, -ene for double, and -yne for triple!
Acronyms
These root words are easy to remember
ME-PB! (Meth-1
Eth-2
Prop-3
But-4).
Flash Cards
Glossary
- Hydrocarbon
An organic compound consisting entirely of hydrogen and carbon atoms.
- Saturated Hydrocarbons
Hydrocarbons that contain only single bonds between carbon atoms.
- Unsaturated Hydrocarbons
Hydrocarbons that contain one or more double or triple bonds.
- Nomenclature
The system of naming chemical substances.
- Alkane
A type of saturated hydrocarbon with the suffix -ane.
- Alkene
A type of unsaturated hydrocarbon with a double bond, indicated by the suffix -ene.
- Alkyne
A type of unsaturated hydrocarbon with a triple bond, indicated by the suffix -yne.
Reference links
Supplementary resources to enhance your learning experience.
