Changes in the State of Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to States and Changes of Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will discuss how matter changes its state. Can anyone tell me how many states of matter we usually discuss?

Three! Solid, liquid, and gas.

Correct! Solid, liquid, and gas are the three primary states. These states can change, and these changes are called physical changes. Does anyone know what a physical change involves?

Is it when the matter changes but still stays the same substance?

Exactly! Physical changes occur without altering the chemical composition. Now, let’s name some common changes in state.

Like melting and freezing?

Yes! Melting is when a solid becomes a liquid. Can anyone give an example of melting?

Like ice turning into water?

Great example! So what happens to the particles during melting?

They move faster and spread apart?

Exactly! Remember this with the acronym 'FAST' - faster, active, spreading out. Let’s summarize: physical changes like melting allow us to witness how temperature can influence state.
Understanding Evaporation and Condensation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about evaporation. Can someone explain what it is?

Isn’t it when a liquid becomes a gas?

Correct! Evaporation occurs mainly at the surface of liquids when they gain enough energy. What about condensation? Who can explain that?

That’s when gas turns into liquid, right?

Exactly! Imagine how steam condenses into water droplets on a cold surface. What variable do you think influences condensation?

The temperature! Lower temperatures help it change from gas to liquid.

Great observation! Let’s remember the relationship with the saying, 'Cool it to condense.' Can anyone provide a real-world example of evaporation?

The puddles drying up after it rains?

Perfect example! To sum it up, both evaporation and condensation are essential for understanding weather patterns.
Sublimation: A Unique Change
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s discuss sublimation. Does anyone know what that is?

I think it’s when a solid turns directly into a gas?

That’s correct! Sublimation skips the liquid state. A common example would be dry ice. Why do you think that happens?

Because the temperature changes too quickly or something?

Exactly! The conditions allow solid molecules to directly enter the gas phase. Remember the mnemonic 'No Liquid Left' to recall sublimation. Can you all think of other examples?

Yeah, like snow turning into water vapor!

Great point! To recap, sublimation is a fascinating change where solids become gases directly.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
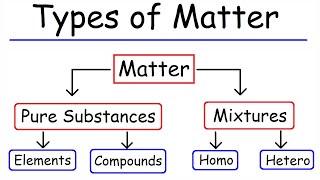
Changes in the state of matter occur as physical transformations influenced by variations in temperature and pressure, including processes such as melting, freezing, evaporation, condensation, and sublimation. Each change involves a reorganization of particles without altering the material's chemical identity.
Detailed
Changes in the State of Matter
In this section, we examine the various physical changes that can occur in matter, emphasizing that these processes do not impact the chemical identity of the substance. The transitions in matter primarily occur due to changes in temperature or pressure, effectively altering particle movement and arrangement. The key transformations discussed include:
- Melting: The process where a solid turns into a liquid as it absorbs heat, increasing the kinetic energy of its particles, which weakens intermolecular forces.
- Freezing: The reverse of melting, where a liquid loses energy and becomes a solid, as its particles slow down and come closer together.
- Evaporation: The transition of a liquid to a gas, typically occurring on the surface of the liquid when it gains sufficient energy.
- Condensation: The change from gas to liquid, often observable when a vapor cools and loses energy, encouraging particle clustering.
- Sublimation: A unique transformation where a solid turns directly into a gas without passing through the liquid phase, seen in certain substances such as dry ice.
Understanding these changes is crucial in chemistry, as it provides insights into the behavior of materials under different conditions.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Physical Changes and Chemical Composition
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Physical changes involve a change in state without altering the chemical composition.
Detailed Explanation
Physical changes refer to changes that occur when a substance changes its state (like from solid to liquid), but its chemical structure remains the same. For example, when ice melts into water, it is still H₂O. The same molecules are present; only their arrangement has changed. This is different from chemical changes, where new substances with different chemical compositions are formed.
Examples & Analogies
Think of a snowman. When the sun comes out, the snowman melts, but it doesn't become a different material—it's just the same snow in liquid form. As soon as the temperature drops, it can freeze back into solid snow again.
Causes of Change of State
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Change of state occurs due to variations in temperature or pressure, affecting particle movement and spacing.
Detailed Explanation
Changes in state happen mainly due to changes in temperature and pressure. For example, heating ice (lowering the temperature) supplies energy to move molecules faster, causing the solid to become a liquid. Conversely, cooling water can slow down the molecules, allowing them to come together and form solid ice. Therefore, temperature directly affects how the particles interact and whether they are tightly packed (solid), loosely arranged (liquid), or far apart (gas).
Examples & Analogies
Consider cooking pasta. When you boil water, the heat makes the water molecules move faster and transition into steam. If you then take the pot off the burner, the steam eventually cools and condenses back into water—showing how temperature can cause substances to change state.
Types of Changes in State
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
○ Melting: Solid to liquid.
○ Freezing: Liquid to solid.
○ Evaporation: Liquid to gas.
○ Condensation: Gas to liquid.
○ Sublimation: Solid to gas without passing through the liquid state.
Detailed Explanation
There are several key processes through which substances change states:
- Melting: When a solid gains enough heat energy, it transitions into a liquid.
- Freezing: When a liquid loses heat energy, it changes back into a solid.
- Evaporation: This happens when a liquid gains enough energy to become a gas; for example, water turning into steam.
- Condensation: This is the reverse process where gas loses energy and becomes a liquid, like water droplets forming on a cold glass.
- Sublimation: This is a unique change where a solid transitions straight to a gas without becoming a liquid first, like dry ice turning into carbon dioxide gas.
Examples & Analogies
Imagine a snowman again: as it warms up (melting), it turns into water. If you put water in the freezer, it becomes ice (freezing). If you leave water in a shallow dish, it eventually disappears as it changes into steam (evaporation). Place your cold drink outside, and you'll see moisture forming on the outside of the glass (condensation). Finally, dry ice is like magic when it goes from solid to fog without a liquid state!
Key Concepts
-
Physical change: A change that doesn’t affect the chemical identity of the substance.
-
Melting: The process where solids become liquids due to heat.
-
Freezing: When liquids turn into solids as they lose heat.
-
Evaporation: The process of a liquid transitioning into gas.
-
Condensation: Transformation of gas into liquid when cooled.
-
Sublimation: The direct change from solid to gas without becoming liquid.
Examples & Applications
Melting of ice into water.
Freezing of water to form ice.
Evaporation of water from a puddle.
Condensation of water vapor on a cold glass.
Sublimation of dry ice into carbon dioxide gas.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Melting makes a solid wet, warmth's the key, don't forget.
Stories
Imagine a snowman on a sunny day; as it gets warmer, it turns into water, just like ice melts, showing physical changes happening around us.
Memory Tools
'Silly Cows May Eat Cold Strawberries' to remember the state changes: Sublimation, Condensation, Melting, Evaporation, Freezing.
Acronyms
MELT-C
Melting
Evaporation
Liquids (to Gas)
Temperature change
Condensation.
Flash Cards
Glossary
- Physical Change
A transformation of a substance that does not change its chemical composition.
- Melting
The process of a solid turning into a liquid.
- Freezing
The transition from liquid to solid.
- Evaporation
The change of a liquid into a gas.
- Condensation
The process of gas turning into a liquid.
- Sublimation
The transformation of a solid directly into a gas, bypassing the liquid state.
Reference links
Supplementary resources to enhance your learning experience.
