Kinetic Molecular Theory of Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Kinetic Molecular Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to dive into the Kinetic Molecular Theory, which explains how matter behaves at the particle level. Can anyone tell me what matter is made of?

Isn't matter made up of atoms and molecules?

Exactly! Matter consists of tiny particles. Now, can anyone explain the motion of these particles?

They move around randomly, right?

Correct! This constant, random motion is a key point in KMT. The speed of these particles changes with temperature. Who can tell me what happens to particle speed when we heat them?

They move faster!

Great! So, as temperature increases, the kinetic energy rises, meaning particles move faster. Remember, we can use the acronym 'KMT' for Kinetic Molecular Theory to remind us of this important concept.

KMT for Kinetic Molecular Theory!

Exactly! Let's summarize what we discussed: Matter is made of tiny particles that are in constant motion, and their speed increases with temperature.
Understanding Different States of Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's explore how KMT explains the three states of matter: solids, liquids, and gases. What do you know about particles in solids?

They are packed tightly together and can't move much.

Absolutely right! In solids, particles are closely packed and vibrate around fixed positions. How about liquids?

In liquids, they can slide past each other but still stay close.

Yes! Particles in liquids have more space than in solids and can move around each other. Now, who can describe gas behavior?

In gases, particles are far apart and move freely, right?

Exactly! Gas particles are far apart and move independently of one another. Remembering that solids have fixed shape and volume, liquids have definite volume but not fixed shape, and gases have neither is crucial. Let's use the mnemonic 'S-L-G' to remember: Solid-Definite, Liquid-Varies, Gas-Nothing holds!

S-L-G for solid, liquid, gas!

Perfect! To summarize, KMT helps us understand why solids, liquids, and gases behave as they do based on their particle arrangements and motions.
Temperature's Role in Particle Motion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk more about how temperature influences the motion of particles. What happens to particles when we heat a substance?

They speed up!

Exactly! As we heat a substance, its particles gain kinetic energy and move faster. Can anyone provide an example of what happens when we heat ice?

It melts into water!

Right! The solid ice turns into a liquid as the particles gain energy from the heat. Now, if we freeze a liquid, what happens?

It becomes a solid!

Correct! And that’s the role of temperature in changing states. The memory aid we can use here is 'Heat increases, so speed increases' - remember HISS! H for heat, I for increases, S for speed, S for state change!

HISS! Got it!

Great! To sum up, temperature affects the kinetic energy of particles, impacting their motion and states.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines the fundamental postulates of the Kinetic Molecular Theory, focusing on the behavior and interaction of particles in solids, liquids, and gases. As temperature influences the speed of particles, the theory helps in understanding the different states of matter and their corresponding physical properties.
Detailed
Kinetic Molecular Theory of Matter
The Kinetic Molecular Theory (KMT) is a fundamental theory in chemistry that describes the behavior of particles in matter. According to KMT, matter is composed of tiny particles, specifically atoms or molecules, that are in constant and random motion.
Key Postulates:
- Composition of Matter: Matter is made up of very small particles that can be individual atoms or clusters of atoms (molecules).
- Constant Motion: These particles are always in motion, and this motion is random. The speed of this motion, however, is influenced by temperature.
- Effect of Temperature: As the temperature increases, the kinetic energy of the particles also increases, leading to faster movement.
- Inter-particle Forces: The distances between particles and the strengths of the forces acting between them vary significantly in solids, liquids, and gases. For example, particles in solids are tightly packed and vibrate around fixed positions, while those in gases are far apart and move freely with minimal interaction.
Understanding the Kinetic Molecular Theory is essential for explaining various phenomena observed in everyday life, from the melting of ice to the behavior of gases in different temperature conditions.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
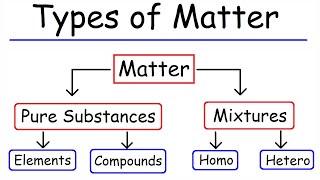
Composition of Matter
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Matter is composed of tiny particles (atoms or molecules).
Detailed Explanation
The Kinetic Molecular Theory tells us that all matter is made up of incredibly small units called particles. These can be atoms, which are the basic building blocks of elements, or molecules, which are made up of two or more atoms bonded together. Understanding that all matter is made of particles helps us grasp how substances interact and change.
Examples & Analogies
Think of atoms like LEGO blocks. Just as many LEGO blocks come together to form different structures (like houses or cars), atoms combine in various ways to create everything around us, from the air we breathe to the water we drink.
Particle Motion
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These particles are in constant, random motion.
Detailed Explanation
One of the key postulates of the Kinetic Molecular Theory is that particles are always on the move. Even if a substance appears solid or stable, the particles are vibrating or moving in some form. This constant motion is fundamental to understanding how matter behaves, particularly how it can change states or react with other substances.
Examples & Analogies
Imagine a crowded dance floor at a wedding. Even when people seem to stay in one place, they are still swaying, spinning, and moving side to side. Similarly, particles in matter are always interacting, moving, and changing their positions, even if their overall form doesn’t change.
Effect of Temperature on Particle Speed
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The speed of particles increases with temperature.
Detailed Explanation
Temperature is a measure of the average kinetic energy of the particles in a substance. As the temperature increases, the particles gain energy and move faster. This higher speed leads to increased interactions among particles and can result in changes of state, like a solid melting into a liquid.
Examples & Analogies
Think about how butter behaves when it's left out at room temperature versus when it's in the fridge. At room temperature, the butter becomes soft and spreadable because the particles gain energy and move more freely. This is similar to how increased temperature affects particle movement in matter.
Spaces and Forces Among Particles
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The spaces between particles and the forces of attraction vary among solids, liquids, and gases.
Detailed Explanation
Different states of matter (solid, liquid, gas) are characterized by the arrangement and behavior of their particles. In solids, particles are closely packed and held together by strong forces, giving them a definite shape and volume. In liquids, particles are still close but can slide past one another, resulting in a definite volume but no fixed shape. In gases, particles are far apart and move freely, having neither definite shape nor volume. This difference in space and force is what defines the behavior of each state of matter.
Examples & Analogies
Think of a crowded room (solid) where people are packed tightly together and can’t move much, a school assembly (liquid) where students are close but can shift in their seats, and an open park (gas) where there’s plenty of space to run around. This shows how the arrangement and attraction of particles differ in various states of matter.
Key Concepts
-
Matter consists of particles that are in constant motion.
-
Temperature affects the speed of particle motion and their kinetic energy.
-
Different states of matter have unique properties based on particle arrangement.
Examples & Applications
When ice melts, it transitions from a solid state with closely packed particles into a liquid state where the particles slide past one another.
As water boils, it changes from a liquid to a gas, with particles gaining enough energy to move freely apart.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Movement is key, particles sway, Solid and liquid, gas on display!
Stories
Imagine a dance party with three groups of friends: the Solids stick together, the Liquids hold hands but can move, and the Gases roam freely all around the room!
Memory Tools
Remember S-L-G for Solid-Liquid-Gas—Solids stay, Liquids play, Gases fly away!
Acronyms
KMT
Kinetic Molecular Theory—K=Kinetic
M=Molecules
T=Theory!
Flash Cards
Glossary
- Kinetic Molecular Theory
A theory that explains the behavior of matter in terms of the motion of its particles.
- Kinetic Energy
The energy that particles possess due to their motion, which increases with temperature.
- Particles
Small units of matter including atoms and molecules that make up substances.
- Solid
A state of matter with a definite shape and volume due to tightly packed particles.
- Liquid
A state of matter with a definite volume but no fixed shape, allowing particles to slide past one another.
- Gas
A state of matter with no definite shape or volume, where particles move freely and are far apart.
Reference links
Supplementary resources to enhance your learning experience.
