Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, class! Today we are discussing matter. Can anyone tell me what matter is?

Isn’t it anything that has mass and takes up space?

Exactly! Matter is indeed anything that has mass and occupies space. Now, can anyone name the three primary states of matter?

Solid, liquid, and gas!

What’s the difference between them?

Great question! Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have neither. Remember: S-L-G! Solids keep their shape, liquids flow, and gases spread out!

Can you give us an example for each?

Of course! An ice cube is a solid, water in a glass is a liquid, and that air we breathe is a gas. Remember these examples—they help solidify the concept!
Kinetic Molecular Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's move on to the Kinetic Molecular Theory of Matter. What do you think it tells us about particles in matter?

That they are always moving?

Exactly! Matter is composed of tiny particles that are always in constant, random motion. Can anybody tell me what happens to these particles when we increase the temperature?

They move faster?

Correct! The speed of particles increases with temperature. The spaces between particles and the forces of attraction differ in solids, liquids, and gases. This explains why they behave differently!

So, gases have the most space?

Exactly! Gases have the largest spaces between particles, which is why they can expand to fill any space. Good job, everyone!
Changes in State
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we'll discuss changes in the state of matter. Who can tell me what a physical change is?

A change that doesn’t change the composition of the substance?

Exactly! Physical changes, such as melting and freezing, occur without altering chemical composition. Can someone give me an example of melting?

Ice melting into water?

Perfect! And what about freezing?

Water turning back into ice!

Great! Other changes include evaporation, which is liquid to gas, and condensation, gas to liquid. Remember: M-F-E-C—Melting, Freezing, Evaporation, Condensation!
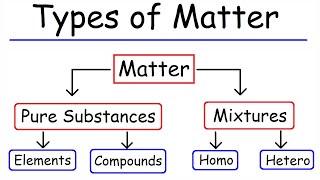
Classification of Matter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s classify matter. Can anyone explain the difference between pure substances and mixtures?

Pure substances are made of one type of atom or molecule, and mixtures are made of two or more!

Correct! Pure substances include elements and compounds, while mixtures can be homogeneous or heterogeneous. Who can give me an example of a homogeneous mixture?

A salt solution?

Exactly! And a heterogeneous mixture?

Sand mixed with iron filings!

Fantastic! Remember: Pure vs. Mixtures. Pure = one type, Mixtures = many types!
Law of Conservation of Mass
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s talk about the Law of Conservation of Mass. Who can explain what it means?

That mass is neither created nor destroyed?

Correct! In a chemical reaction, the total mass of reactants equals the total mass of products. Can anyone give me an example?

When barium chloride and sodium sulfate react, the total mass before and after stays the same.

Exactly! Understanding these principles is crucial in chemistry, as they guide how we study and experiment with matter.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section introduces the concept of matter, discusses the Kinetic Molecular Theory, explains changes in states, and covers the classification of matter, including pure substances and mixtures. It also touches on physical and chemical changes, the Law of Conservation of Mass, and the importance of studying matter.
Detailed
Chapter 1: Matter
Overview
This chapter introduces the concept of matter, defined as anything that has mass and occupies space. Matter exists primarily in three states: solids, which have a definite shape and volume; liquids, which have a definite volume but no fixed shape; and gases, which have neither fixed shape nor volume. Understanding these states sets the foundation for further exploration into the properties and behaviors of different materials.
Kinetic Molecular Theory
The Kinetic Molecular Theory explains that matter is composed of tiny particles (atoms or molecules) that are in constant, random motion. The speed of these particles increases with temperature, and their interactions vary in strength, leading to different states of matter.
Changes in State
Physical changes, such as melting and evaporation, occur without altering the chemical composition of the substance. These changes are driven by variations in temperature and pressure, affecting particle movement. Other changes include freezing, condensation, and sublimation, which describe the transitions between solid, liquid, and gas states.
Law of Conservation of Mass
This law states that mass is neither created nor destroyed during a chemical reaction, meaning the total mass of reactants equals that of products. This foundational principle underscores the importance of matter in chemical processes.
Classification of Matter
Matter is classified into pure substances (elements and compounds) and mixtures (homogeneous and heterogeneous). Understanding these classifications is essential for grasping the nature of different materials.
Physical and Chemical Changes
Physical changes do not create new substances and can be reversed easily, while chemical changes result in new substances and are usually irreversible. Recognizing these differences is crucial in chemistry.
Separation Techniques
Various techniques are used to separate mixtures, including filtration, evaporation, distillation, chromatography, and magnetic separation, each suited for specific types of materials.
Importance of Studying Matter
Studying matter is fundamental to chemistry and has practical applications in various fields, from pharmaceuticals to material science.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Matter
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Matter is anything that has mass and occupies space.
● It exists in three primary states:
○ Solid: Definite shape and volume.
○ Liquid: Definite volume but no definite shape.
○ Gas: Neither definite shape nor volume.
Detailed Explanation
Matter is defined as anything that has mass (weight) and takes up space (volume). This means all physical substances, from the air we breathe to the water we drink, are considered matter. Matter can exist in three main states: solid, liquid, and gas. In a solid, particles are tightly packed, giving it a fixed shape and volume. In a liquid, while the volume remains constant, the shape can change depending on the container. In a gas, both shape and volume can change because the particles are far apart and move freely.
Examples & Analogies
Think of matter like ingredients for a recipe. Just as flour, sugar, and eggs can take different forms (like cake, cookies, or bread), matter can take different states. Imagine butter as a solid when it's cold (like when you take it out of the fridge). When you melt it, it becomes a liquid, and when you heat it further, it can turn into a gas (steam)!
Kinetic Molecular Theory of Matter
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Postulates:
○ Matter is composed of tiny particles (atoms or molecules).
○ These particles are in constant, random motion.
○ The speed of particles increases with temperature.
○ The spaces between particles and the forces of attraction vary among solids, liquids, and gases.
Detailed Explanation
The Kinetic Molecular Theory explains that all matter is made up of very small particles called atoms or molecules. These particles are never at rest; they are always moving randomly. The speed at which these particles move is affected by temperature: when temperature goes up, particles move faster. Additionally, the distance between these particles and the attractive forces between them differ based on whether the matter is a solid, liquid, or gas, which explains the different properties of these states.
Examples & Analogies
Imagine a room filled with people dancing at different speeds. In a crowd (gas), people are moving quickly and can bump into each other. If the room quiets down (cool temperature), they start moving closer together but still have some space to move (liquid). Finally, in a tightly packed elevator (solid), everyone is standing still and fits closely together. This illustrates how particle movement and spacing change with matter states!
Changes in the State of Matter
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Physical changes involve a change in state without altering the chemical composition.
● Change of state occurs due to variations in temperature or pressure, affecting particle movement and spacing.
○ Melting: Solid to liquid.
○ Freezing: Liquid to solid.
○ Evaporation: Liquid to gas.
○ Condensation: Gas to liquid.
○ Sublimation: Solid to gas without passing through the liquid state.
Detailed Explanation
Changes in the state of matter are categorized as physical changes because they do not affect the chemical composition of the substance involved. These changes happen when temperature or pressure varies, which alters how the particles move and how close they are to each other. For example, when ice (solid) warms up enough, it melts into water (liquid). Conversely, if water is cooled, it freezes back into ice. Other changes include evaporation, where liquid water becomes gas (steam), and condensation, where gas turns back into liquid water.
Examples & Analogies
Think about making ice cream. You start with a liquid mixture (cream and sugar). When you cool it down and churn it (change in temperature and pressure), it changes to a solid (ice cream). Each step back and forth, whether from liquid to solid or solid to gas, shows how matter changes state!
Key Concepts
-
Matter: Anything that has mass and occupies space.
-
Three States of Matter: Solid, liquid, and gas.
-
Kinetic Molecular Theory: Matter is composed of particles in constant motion.
-
Physical Changes: Changes in state without altering composition.
-
Chemical Changes: Changes that result in new substances.
-
Law of Conservation of Mass: Mass is conserved in reactions.
-
Classification of Matter: Pure substances versus mixtures.
Examples & Applications
Ice melting into water is a physical change.
Rust forming on iron is a chemical change.
A salt solution is a homogeneous mixture.
Sand and iron filings is a heterogeneous mixture.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Matter's anything with mass, shape and space, it will always amass.
Stories
Once upon a time, there were three friends: Solid, the strong and sturdy one; Liquid, who loved to flow and adapt; and Gas, who was free and unrestricted. Together, they made up all of matter, but in different ways!
Memory Tools
To remember states of matter: S-L-G (Solid-Liquid-Gas).
Acronyms
Use the acronym M-F-E-C for changes in state
Melting
Freezing
Evaporation
Condensation.
Flash Cards
Glossary
- Matter
Anything that has mass and occupies space.
- Solid
A state of matter with a definite shape and volume.
- Liquid
A state of matter with a definite volume but no definite shape.
- Gas
A state of matter with neither a definite shape nor volume.
- Kinetic Molecular Theory
A theory that explains the behavior of matter in terms of particles in motion.
- Physical Change
A change in the state of matter without altering its chemical composition.
- Chemical Change
A process where a substance transforms into a new substance.
- Law of Conservation of Mass
The principle that mass is neither created nor destroyed in a chemical reaction.
- Pure Substance
Matter that has a uniform and definite composition.
- Mixture
A combination of two or more substances that retains their individual properties.
Reference links
Supplementary resources to enhance your learning experience.
