Physical and Chemical Changes
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Physical Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're learning about physical changes. Who can tell me what a physical change is?

Is it when something changes without becoming a new substance?

Exactly! Physical changes don't create new substances, they just change the appearance or state. Can anyone give an example?

Melting ice! When ice melts, it becomes water but it’s still H2O!

Great example! You can also dissolve sugar in water, right? It’s still sugar even when dissolved.

So, both are reversible changes?

Exactly, since you can freeze water back into ice! Let’s remember physical changes with the acronym ‘PRAISE’, standing for 'Physical Reversible Appearance Is Still the same Entity'.
Exploring Chemical Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s shift to chemical changes. What happens during a chemical change?

New substances are formed, right?

That’s correct! And what are some signs that a chemical change has occurred?

Like color change or gas being produced?

Exactly! And temperature changes can also indicate a chemical reaction. Can anyone think of an example?

Rusting iron is a good example. It changes color and gets flaky.

Well done! Let’s use the mnemonic ‘CREDITS’ to remember chemical changes: 'Chemical Reaction Emergence, Different, Irreversible Transformation of Substances'.
Comparison of Physical and Chemical Changes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s recap what we’ve learned. How do physical and chemical changes differ?

Physical changes don’t create new substances and are reversible, whereas chemical changes do create new substances and are often irreversible.

Perfect summary! Why is this understanding important in chemistry?

It helps us understand how materials interact and change in reactions!

Exactly! Knowing this helps in fields like material science and environmental studies. Remember to use ‘PRAISE’ and ‘CREDITS’ as your memory aids!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we differentiate between physical and chemical changes. Physical changes involve no new substance formation and are typically reversible, while chemical changes result in new substances and are often irreversible. Examples such as melting ice and rusting iron illustrate these concepts.
Detailed
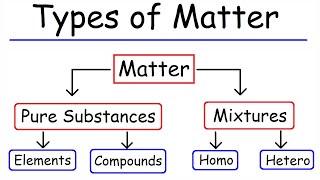
Physical and Chemical Changes
In this section, we explore the concepts of physical and chemical changes crucial to understanding matter's behaviors:
- Physical Changes:
- These changes do not create new substances. Instead, they alter the state or appearance of a substance without modifying its chemical composition.
- Physical changes are generally reversible; examples include the melting of ice and dissolving sugar in water.
- Chemical Changes:
- Chemical changes, in contrast, result in the formation of new substances with distinct properties. These changes typically involve a chemical reaction, which alters the atomic and molecular structure.
- Indicators of chemical changes can include color changes, gas production, and temperature changes. Common examples include rusting of iron and combustion of paper.
Understanding these differences is vital for recognizing how substances interact and transform in various contexts, laying a foundational aspect of the study of chemistry.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Physical Changes
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Physical Changes:
- No new substance is formed.
- Reversible changes.
- Examples: Melting of ice, dissolving sugar in water.
Detailed Explanation
Physical changes are alterations that do not affect the chemical composition of a substance. During a physical change, the original substance remains the same; it simply undergoes a transformation in state, appearance, or phase. For instance, when ice melts into water, it is still H2O, just in a different state. Additionally, many physical changes are reversible, meaning that you can easily return the substance to its original form. For example, if you freeze water into ice, you can melt it back into water again.
Examples & Analogies
Think about ice cream. When you take it out of the freezer, it starts to melt. This is a physical change because it's still ice cream, just in a different state (liquid instead of solid). If you put it back in the freezer, it turns back into solid ice cream, showing the reversible nature of physical changes.
Chemical Changes
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Chemical Changes:
- New substances are formed.
- Usually irreversible.
- Indicators include color change, gas production, and temperature change.
- Examples: Rusting of iron, burning of paper.
Detailed Explanation
Chemical changes result in the formation of new substances. Unlike physical changes, these changes involve a rearrangement of atoms and bonds, resulting in substances that have completely different properties from the original materials. These changes are often irreversible; for example, when paper burns, it transforms into ash and gases that cannot be converted back into paper. Indicators of chemical changes can include color changes, the production of gas (like bubbles), and changes in temperature (either heat being absorbed or released).
Examples & Analogies
Imagine baking a cake. When you mix flour, sugar, eggs, and other ingredients, and then bake it, a chemical change occurs. The mixture transforms into a cake, which cannot be turned back into its original ingredients. This is because new substances are created through the change, represented by the delicious cake you can now eat!
Key Concepts
-
Physical Changes: Changes that do not alter the chemical composition of substances.
-
Chemical Changes: Changes that result in new substances being formed.
Examples & Applications
Melting of ice into water (Physical Change)
Rusting of iron (Chemical Change)
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Freeze to ice, melt to water, physical changes make things alter.
Stories
Once upon a time, ice decided to melt into water. It loved being liquid, but when it froze again, it felt like itself once more. Yet, when iron rusted, it changed its nature entirely, like a caterpillar becoming a butterfly!
Memory Tools
CAGED for Chemical Changes: Color change, Ash formation, Gas production, Energy change, Distinct new substance.
Acronyms
PRAISE for Physical Changes
Physical Reversible Appearance Is Still the same Entity.
Flash Cards
Glossary
- Physical Change
A change that alters the form or appearance of a substance without changing its chemical composition.
- Chemical Change
A change that results in the formation of one or more new substances with different properties.
Reference links
Supplementary resources to enhance your learning experience.
