Pressure Variation with Depth in Fluids
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Pressure Variation with Depth
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re discussing how pressure varies with depth in fluids. As you go deeper into a liquid, what happens to the pressure?

The pressure increases, right?

Exactly! The formula we can use to calculate pressure at different depths is P = ρgh. Can anyone tell me what each symbol represents?

P is pressure, ρ is the density of the liquid, g is gravitational acceleration, and h is the depth.

Great job! Now, let's explore how the density of different liquids affects pressure at the same depth.
Pressure Comparison Across Different Liquids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

If we look at depth pressure, say, 10 meters deep, how would pressure in water compare to pressure in oil?

Water would have higher pressure because it’s denser.

Correct! Oil has a lower density than water, leading to lower pressure at the same depth. Let's review why this matters.

So if I'm diving into an oil pool, I wouldn’t feel as much pressure compared to diving into water!

That's right! Densities can greatly affect how we experience pressure underwater.
Summary of Key Points
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s summarize. What happens to pressure as depth increases and how does the density of the fluid affect pressure?

Pressure increases with depth, and denser liquids have higher pressures at the same depth.

Excellent! Remember, this concept is critical in understanding fluid dynamics and applications in real life, such as underwater exploration and engineering.

So pressure gauges also factor in fluid density when measuring pressure?

Spot on! Always keep density in mind when comparing pressures across different fluids.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
As we dive deeper into a fluid, the pressure rises proportionally with depth. This section compares the pressure at different depths in various fluids, highlighting how density affects pressure readings.
Detailed
Pressure Variation with Depth in Fluids
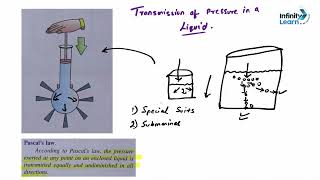
Pressure in a fluid increases with depth due to the weight of the fluid above the measurement point. The underlying concept is that at greater depths, there is more liquid pressing down, leading to increased pressure. The formula that defines this relationship is given by:
P = ρgh
Where:
- P = Pressure (Pa)
- ρ = Density of the fluid (kg/m³)
- g = Gravitational acceleration (approximately 9.8 m/s² on Earth)
- h = Depth in the fluid (m)
Furthermore, the difference in fluid densities plays a vital role: for instance, water has a density of 1000 kg/m³, therefore at a specific depth, the pressure would be different in oil (which has a lower density) as compared to mercury (which has a higher density). This section emphasizes the direct correlation between pressure and density in determining fluid pressure at various depths.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Pressure at Different Depths
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As we move deeper into a liquid, the pressure increases. The pressure at a point below the surface of the liquid is proportional to the depth of the point.
Detailed Explanation
This chunk explains how pressure behaves as we go deeper into a liquid. As we descend deeper, the pressure consistently rises. This increase in pressure happens because there is more liquid above us pushing down due to gravity. For instance, when you dip into a swimming pool, you’ll notice that the water feels heavier on your body the deeper you go—which is the water's pressure increasing at that depth.
Examples & Analogies
Imagine stacking books on a table. If you place one book, there isn't much weight on the table, but if you add ten books, the pressure on the table increases significantly because of the weight of all the books stacked on top.
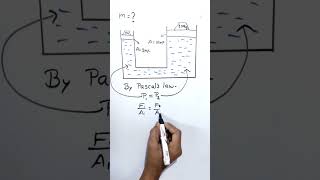
Pressure at a Given Depth in Different Liquids
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The pressure at a given depth in different liquids depends on their densities. Since water has a density of 1000 kg/m³, the pressure at a given depth in oil (which is less dense) will be lower than in water, and the pressure in mercury (which is denser) will be higher.
Detailed Explanation
Here, the focus is on how the density of different liquids affects pressure at the same depth. All fluids experience pressure due to their weight, but the amount of pressure depends on how dense the fluid is. For example, at a specific depth, water, oil, and mercury will exert different pressures because they have different densities. Water exerts a certain pressure at 10 meters; oil, being less dense, will exert less pressure at the same depth, while mercury, being denser, will exert more.
Examples & Analogies
Consider filling different containers—one with water, one with vegetable oil, and one with honey. If you poke a hole in the side of each container at the same depth, the liquid with the highest density (like honey) will push out and flow the fastest, while the lighter liquid (like oil) will flow more slowly. This illustrates how density affects pressure.
Key Concepts
-
Pressure increases with depth: As you dive deeper into a liquid, the pressure increases linearly with distance under the surface.
-
Impact of Density: Different fluids have different densities that directly affect the pressure at a specific depth.
Examples & Applications
At a depth of 5 meters in water, the pressure is approximately 49,000 Pa, since water has a density of 1000 kg/m³.
In mercury, pressure at the same depth would be higher due to its higher density, illustrating that denser fluids exert more pressure.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
As you dive down, pressure goes up, like the liquid fills a cup.
Stories
Imagine diving into a pool with no bottom. The deeper you go, the more water weighs on your body, pushing you down.
Memory Tools
D.P.D. - Depth Provokes Density; remember that pressure depends on density and depth!
Acronyms
D.P.E. - Depth Pressure Equation
Pressure = Density x Gravity x Depth.
Flash Cards
Glossary
- Pressure
The force exerted by a fluid per unit area.
- Depth
The distance below the surface of a fluid.
- Density
The mass per unit volume of a substance, typically expressed in kg/m³.
- Hydrostatic Pressure
The pressure exerted by a fluid at rest due to the weight of the fluid above it.
Reference links
Supplementary resources to enhance your learning experience.
