Ozone Depletion
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are discussing the ozone layer. Can anyone tell me what the ozone layer does?

It protects us from the Sun's harmful UV radiation!

Exactly! The ozone layer absorbs most of the Sun's UV radiation, which is crucial for our health. Can anyone think of why that might be important?

Because too much UV can cause skin cancer?

Right! Skin cancer is one major risk from increased UV levels. Let's remember this using the acronym UV for 'Ultra-Violent' rays, as those rays are what we want to block!
Human Impact on Ozone Layer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What do you think are some human activities that harm the ozone layer?

Using sprays that contain CFCs?

Exactly! Chlorofluorocarbons, or CFCs, are found in aerosols and refrigerants. CFCs release chlorine when they break down, and chlorine is harmful to ozone. Let's think of CFCs as 'Chemicals For Clouds' since they contribute to ozone depletion.

Are there other things besides CFCs?

Yes, there are other ozone-depleting substances as well, but CFCs are the most common. Understanding these terms is vital, especially when we discuss international regulations.
Consequences of Ozone Depletion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What are some consequences we face due to ozone layer depletion?

More skin cancer and cataracts, right?

Yes, and it also affects ecosystems. Higher UV levels can damage marine life and reduce agricultural yields. We can visualize this by thinking of a 'Sun's Wrath' that affects not just humans but also nature.

What can we do to help this situation?

Great question! International agreements like the Montreal Protocol are addressing these issues. Always remember that even small changes in our use of products can help reduce ozone depletion.
Efforts to Mitigate Ozone Depletion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone name an international effort to protect the ozone layer?

The Montreal Protocol!

Correct! The Montreal Protocol is significant as it aims to phase out CFCs and other harmful substances. Let's remember this by saying, 'Montreal Means Safety for Ozone'.

Is it actually working?

Yes, studies show that the ozone layer is slowly recovering, but we must continue our efforts. Sustainable actions are necessary for long-term protection.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The depletion of the ozone layer, primarily due to chlorofluorocarbons (CFCs) and other ozone-depleting substances, poses significant risks to human health and ecosystems. The ozone layer plays a crucial role in protecting the Earth from harmful ultraviolet (UV) radiation.
Detailed
Ozone Depletion
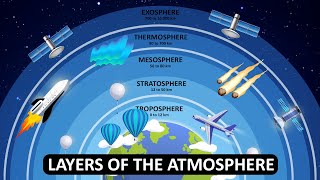
Ozone depletion refers to the thinning of the ozone layer in the stratosphere, a phenomenon primarily driven by human activities. The ozone layer is essential in absorbing and blocking harmful ultraviolet (UV) radiation from the Sun. Without this protective layer, there would be an increase in UV radiation reaching the Earth's surface, leading to an array of health issues such as skin cancer, cataracts, and immune system suppression.
Causes of Ozone Depletion
The main contributors to ozone depletion are chlorofluorocarbons (CFCs), halons, and other ozone-depleting substances (ODS). These chemicals release chlorine and bromine when they are broken down by UV light in the atmosphere, which in turn catalyzes the destruction of ozone molecules.
Health and Environmental Implications
The thinning of the ozone layer not only increases the risk of skin cancer and cataracts but also negatively impacts ecosystems, particularly marine life, as UV radiation affects phytoplankton, the foundation of the marine food web. Plants and animals on land can also suffer from higher UV exposure, leading to reduced agricultural yields and biodiversity loss.
Mitigating Ozone Depletion
International initiatives such as the Montreal Protocol have been critical in phasing out the use of CFCs and other ODS, demonstrating a successful global response to the issue of ozone depletion. Continued efforts to monitor and regulate substances harming the ozone layer are essential for its recovery and protection of life on Earth.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Ozone Depletion
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The use of chlorofluorocarbons (CFCs) and other ozone-depleting substances has led to the thinning of the ozone layer, particularly in the polar regions.
Detailed Explanation
Ozone depletion refers to the reduction of the ozone layer, which is a part of the Earth's stratosphere that absorbs most of the Sun's harmful ultraviolet (UV) radiation. Chlorofluorocarbons (CFCs) and other chemicals are the main culprits behind this thinning. When these substances are released into the atmosphere, they rise to the stratosphere where they break down ozone (O3) molecules. As a result, there are fewer ozone molecules left to protect the Earth from UV radiation, particularly in colder regions like the poles, leading to increased exposure.
Examples & Analogies
Imagine the ozone layer as a protective sunscreen for the Earth. If we keep using products that contain CFCs, it’s like wiping off our sunscreen every day, making our skin more vulnerable to sunburns. Just as too much sun can cause skin problems, the depletion of ozone can lead to harmful effects like skin cancer and eye cataracts.
Importance of the Ozone Layer
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ozone layer protects life on Earth from harmful ultraviolet radiation, and its depletion has increased the risks of skin cancer, cataracts, and other health issues.
Detailed Explanation
The ozone layer serves as a shield, absorbing the majority of the Sun’s dangerous UV rays. This protection is crucial for living organisms including humans, animals, and plants. Increased UV exposure due to ozone depletion has been linked to several health concerns, particularly skin cancers and eye damage such as cataracts. Furthermore, UV radiation can adversely affect ecosystems, reducing biodiversity and disrupting food chains.
Examples & Analogies
Think of the ozone layer like a magician's curtain that blocks harmful rays from the spotlight (the Sun) during a performance. If the curtain is damaged and torn, the harsh lights will shine through, blinding the performers (life on Earth). Similarly, when the ozone layer gets thinner, it lets more harmful UV rays reach us.
Key Concepts
-
Ozone Layer: A protective layer in the atmosphere that absorbs UV radiation.
-
CFCs: Substances that contribute to ozone depletion by releasing chlorine.
-
Health Risks: Increased UV radiation leads to health issues like skin cancer.
-
Global Initiatives: Efforts like the Montreal Protocol aim to reduce ozone-depleting substances.
Examples & Applications
The rise in skin cancer rates in regions with high UV exposure due to ozone depletion.
The success of the Montreal Protocol in reducing global CFC usage.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ozone layer, keep us fair, from the Sun's UV scare.
Stories
Once upon a time, the Earth was protected by a magical layer that kept the bad UV rays at bay, but mischievous CFC sprites started breaking it down, causing the land to suffer—now all of nature works together to save the magical layer.
Memory Tools
CFC = Chemicals For Clouds, as they contribute to ozone depletion.
Acronyms
SAVE - 'Stop Added Variables for the Environment' to remember to reduce ozone-depleting substances.
Flash Cards
Glossary
- Ozone Layer
A region of Earth's stratosphere that absorbs most of the Sun's harmful ultraviolet radiation.
- Chlorofluorocarbons (CFCs)
Chemical compounds used in aerosols, refrigerators, and air conditioners that deplete the ozone layer.
- Ultraviolet (UV) Radiation
A form of radiation from the Sun that can cause skin cancer and cataracts.
- Montreal Protocol
An international treaty designed to phase out substances that deplete the ozone layer.
Reference links
Supplementary resources to enhance your learning experience.
