Extracting Metals towards the Top of the Activity Series
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Reactive Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will learn about extracting highly reactive metals. Can anyone tell me what makes a metal 'reactive'?

I think it means the metal can easily react with other substances.

Excellent! Metals like sodium and magnesium are so reactive that we can't extract them using carbon. Why do you think that is?

Maybe because they are too strong and would rather react with oxygen than carbon?

Yes, they have a greater affinity for oxygen!

Exactly! So, to extract these metals, we use a method called electrolytic reduction. Let's break that down.
Electrolytic Reduction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In electrolytic reduction, we pass an electric current through a molten solution of the metal's compound. Can anyone guess what happens at the electrodes?

The metal gets deposited at the negative electrode!

Right! And what about the positive electrode?

That's where the non-metals go, like chlorine gas if it’s sodium chloride.

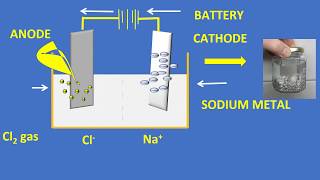
Perfect! For sodium, at the cathode, we have Na⁺ + e⁻ → Na. And at the anode, 2Cl⁻ → Cl₂ + 2e⁻. Could anyone remember what we get from magnesium and aluminum?

They would follow a similar process, right?

Yes! Magnesium is extracted from its chloride, and aluminum from its oxide. It’s all about separating the metal from its compound! Let's dive a bit deeper into the aluminum process next.
Applications of Extracted Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've learned about the extraction processes, why do you think extracting aluminum and sodium is important?

Aluminum is used everywhere from cans to airplanes!

And sodium is important for things like batteries!

Exactly! These metals play crucial roles in various industries. After knowing how they are extracted, do you think their properties make them suitable for these applications?

Definitely! Aluminum is lightweight and corrosion-resistant.

Great observations! Understanding these processes not only helps in metallurgy but also in appreciating their applications in real life. Before we conclude, who remembers the basic reaction happening during the extraction?

The metal ions gain electrons and get reduced!

Spot on! That's the essence of electrolytic reduction, well done!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains that metals at the top of the activity series, such as sodium and aluminum, cannot be extracted using carbon due to their high reactivity. Instead, electrolytic reduction is used to obtain these metals from their compounds. The section also provides specific examples of metals extracted through this process and outlines the reactions involved.
Detailed
Extracting Metals High in the Activity Series
The extraction of metals from their ores varies significantly based on their position in the activity series. Metals that are placed towards the top of the activity series are highly reactive and cannot be obtained from their compounds through reduction using carbon. This is mainly because these metals, including sodium, magnesium, calcium, and aluminum, have a stronger affinity for oxygen compared to carbon.
Electrolytic Reduction Process
To extract these highly reactive metals, electrolytic reduction is employed. In this process, molten salts of the metals are electrolyzed, leading to the deposition of the metal at the cathode and the liberation of non-metallic gases at the anode. For instance, sodium is extracted from molten sodium chloride, magnesium from molten magnesium chloride, and aluminum from molten aluminum oxide.
- For Sodium:
- At the cathode: Na⁺ + e⁻ → Na
- At the anode: 2Cl⁻ → Cl₂ + 2e⁻
- For Aluminum:
- The process involves melting aluminum oxide (Al₂O₃) and performing electrolysis to separate aluminum from oxygen.
These methods are crucial not just for metallurgical processes but also for various industrial applications where purity and high reactivity are vital.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Reactivity of Metals in the Activity Series
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The metals high up in the reactivity series are very reactive. They cannot be obtained from their compounds by heating with carbon. For example, carbon cannot reduce the oxides of sodium, magnesium, calcium, aluminium, etc., to the respective metals. This is because these metals have more affinity for oxygen than carbon.
Detailed Explanation
Metals in the reactivity series are categorized based on their ability to displace other metals from compounds or their stability when combined with oxygen. The higher the metal is on the list, the more reactive it is. Metals like sodium, magnesium, calcium, and aluminium can't be extracted just by heating with carbon because they react more readily with oxygen than carbon does. So, we need different methods to extract these metals from their ores. Essentially, their high reactivity means they prefer to exist as bonded compounds rather than as free metals.
Examples & Analogies
Think of it like a game of musical chairs. The more popular chairs (metals) get filled quickly, and less popular ones (like carbon) are left empty. In our case, the 'popular chairs' (reactive metals) do not leave their seats for carbon to take their place. They are more comfortable remaining paired with oxygen, thus making them challenging to extract.
Electrolytic Reduction
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These metals are obtained by electrolytic reduction. For example, sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides. The metals are deposited at the cathode (the negatively charged electrode), whereas, chlorine is liberated at the anode (the positively charged electrode). The reactions are – At cathode Na+ + e– → Na At anode 2Cl– → Cl + 2e–
Detailed Explanation
Electrolytic reduction is a method used to extract highly reactive metals. In this process, we use electricity to drive the reaction that separates these metals from their compounds. For instance, when electrolyzing molten sodium chloride, sodium ions (Na+) gain electrons at the negatively charged electrode (cathode) to become sodium metal, while chloride ions (Cl–) lose electrons at the positively charged electrode (anode) to produce chlorine gas. This is an efficient way of isolating metals that don’t give up their oxygen easily.
Examples & Analogies
Imagine you have a stubborn friend (the metal) who won’t leave a party (compound) until you promise them fun (electrical energy). You can’t just call out to them (use carbon); instead, you have to offer them something exciting (electrical current) to entice them to come out. Just as friends need motivation to leave a dull gathering, these metals need an electrical push to break free from their compounds.
Electrolysis of Aluminium Oxide
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Similarly, aluminium is obtained by the electrolytic reduction of aluminium oxide.
Detailed Explanation
Aluminium oxide is also extracted through electrolytic reduction. In a similar manner as with sodium, we apply electricity to separate aluminium metal from its oxide. The process occurs in a molten state and requires careful management of temperature and electrical current to optimize the yield of pure aluminium.
Examples & Analogies
Think of baking a cake. Just as you combine the right ingredients (your starting compounds) and bake them at the right temperature to get your delicious cake (pure aluminium), you similarly control the conditions in electrolytic reduction to get high-purity aluminium. Every step in the process is crucial to ensure you get what you want without burning the cake.
Key Concepts
-
Electrolytic Reduction: A critical process for extracting metals with high reactivity by using electric current.
-
Activity Series: A ranking of metals based on their reactivity, which impacts extraction methods.
-
Affinity for Oxygen: Highly reactive metals have a stronger tendency to combine with oxygen than to be reduced by carbon.
Examples & Applications
For sodium extraction, the reaction at the cathode is Na⁺ + e⁻ → Na, and at the anode, 2Cl⁻ → Cl₂ + 2e⁻.
Aluminum is extracted from its oxide using electrolytic reduction where aluminum ions gain electrons to form aluminum metal.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To extract the metal, turn up the heat, or electrolyze for those we can't meet!
Stories
Imagine a factory where superheroes, the metals, wait for their turn. The high-flying sodium can only be freed through a power bolt, not a carbon hug!
Memory Tools
For extracting sodium and magnesium, think 'ELECTRO-' for Electrolytic process.
Acronyms
RAM
Reactive metals require electrolytic methods.
Flash Cards
Glossary
- Electrolysis
A process that uses electrical energy to cause a chemical change, typically to separate elements from their compounds.
- Affinity for Oxygen
The tendency of a substance to react with oxygen, often resulting in the formation of oxides.
- Electrolytic Reduction
The process of using electric current to reduce metal ions into pure metal.
Reference links
Supplementary resources to enhance your learning experience.
