What happens when Metals are burnt in Air?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Metal Reactions in Air
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to learn what happens when various metals are burnt in the air. Can anyone tell me what they think happens during this process?

I think they might change into dust or something like that!

Interesting thought! Most metals actually react with oxygen to form metal oxides. This means their chemical structure changes.

So, what does that look like visually when you burn them?

Great question! For example, magnesium burns brightly to form magnesium oxide. It’s like fireworks. Does anyone know what color flame it produces?

A bright white flame!

Exactly! Remember, metals like magnesium react vigorously with oxygen. Now, let's recap: metals react with oxygen to form oxides.
Reactivity of Different Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about how different metals react at varying levels. Who can name a metal that burns easily in the air?

Is sodium one of them? I think it reacts super strongly with air.

Correct! Sodium reacts violently; that’s why we keep it in oil. Which metals do you think don’t react much at all?

Gold and silver, right? They don’t seem to change with heat.

Absolutely right! These noble metals hardly react with oxygen. It's vital to note these differences in reactivity.

So we could create a list of metals based on how reactive they are? Like a ranking?

Exactly! That's our reactivity series. As a takeaway, remember: metals vary a lot in how they interact with oxygen.
Formation of Amphoteric Oxides
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's dive into what we call amphoteric oxides. Can anyone guess what that term might mean?

Maybe oxides that can act as both acids and bases?

Spot on! For example, aluminum oxide can react with both acids and bases. Does anyone know the reactions it undergoes?

With hydrochloric acid, it forms salts!

Correct! And this dual behavior is significant in various chemical processes. So, in summary, amphoteric oxides play a diverse role.
Protective Layers on Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss how some metals are protected by a thin oxide layer. Can anyone give me an example of a metal that doesn’t rust because of this?

Aluminum! I read that it develops this layer when exposed to air.

Absolutely! This layer shields the metal from further oxidation. Isn’t that fascinating?

How about iron? Doesn’t it rust instead of forming a protective layer?

Good observation! Iron forms rust and doesn't develop the protective layer like aluminum. Remember, protective layers can be a metal’s best friend.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section outlines the burning process of metals in the presence of air, illustrating that most metals react with oxygen to form metal oxides. It emphasizes the differences in reactivity, citing specific examples, and introduces important concepts like amphoteric oxides.
Detailed
In this section, we explore the reaction of metals with oxygen when burnt in air. Most metals react with oxygen, producing metal oxides. For instance, magnesium burns with a bright white flame to form magnesium oxide, while copper burns to form copper(II) oxide, which is black. The reactivity of metals varies significantly; potassium and sodium react vigorously, while silver and gold are unreactive even at high temperatures. The metals can be arranged in a reactivity series based on their behavior in the reaction with oxygen. Most metal oxides are basic, but some, like aluminum oxide, show amphoteric behavior, reacting both with acids and bases. The section concludes by noting protective oxide layers that form on certain metals, preventing further oxidation, and underscores distinct characteristics among metals during oxidation.
Youtube Videos






![🔥What Happens When Metals React With Water?🔥[Mg + H2O] | Class 10 Science Chapter 3](https://img.youtube.com/vi/HN72_gAlnpE/mqdefault.jpg)

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Metal Combustion
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You have seen in Activity 3.8 that magnesium burns in air with a dazzling white flame. Do all metals react in the same manner? Let us check by performing the following Activity.
Detailed Explanation
This introductory statement sets the stage for exploring how different metals behave when they are burned in air. It prompts curiosity about whether all metals will react similarly to oxygen in the air, starting with magnesium as a clear example of a metal that reacts dramatically.
Examples & Analogies
Think of firecrackers: just like they produce different colors and sounds when ignited, different metals can produce varying reactions when burned in the air.
Experimental Activity
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
CAUTION: The following activity needs the teacher’s assistance. It would be better if students wear eye protection. n Hold any of the samples taken above with a pair of tongs and try burning over a flame. Repeat with the other metal samples. n Collect the product if formed. n Let the products and the metal surface cool down. n Which metals burn easily? n What flame colour did you observe when the metal burnt? n How does the metal surface appear after burning? n Arrange the metals in the decreasing order of their reactivity towards oxygen. n Are the products soluble in water?
Detailed Explanation
This section outlines an experimental activity to observe how various metals react with oxygen. It highlights the importance of safety precautions and gives a structured approach: burn the metals, collect the products, observe the flame color, assess reactivity, and check product solubility. This step-by-step guidance helps students learn through direct observation and experimentation.
Examples & Analogies
Imagine conducting a science experiment akin to a cooking show. Each metal is like a different ingredient, and by 'cooking' them with flame, students get to see how they transform, similar to how ingredients react when heated.
Formation of Metal Oxides
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Almost all metals combine with oxygen to form metal oxides. Metal + Oxygen → Metal oxide. For example, when copper is heated in air, it combines with oxygen to form copper(II) oxide, a black oxide. 2Cu + O → 2CuO (Copper) (Copper(II) oxide). Similarly, aluminium forms aluminium oxide. 4Al + 3O → 2Al2O3 (Aluminium) (Aluminium oxide).
Detailed Explanation
This section describes the chemical reaction that occurs when metals burn in air. It explains that metals generally react with oxygen to create metal oxides, illustrating the concept with copper and aluminum as examples. The provided chemical equations reinforce how elements combine during combustion.
Examples & Analogies
Think of a metal like a friend who loves to team up with others. Just like how friends can form a strong group to achieve something great together, metals 'team up' with oxygen to form new substances (metal oxides) that have different properties than the metals alone.
Properties of Metal Oxides
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
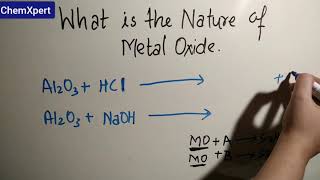
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We have learnt that metal oxides are basic in nature. But some metal oxides, such as aluminium oxide, zinc oxide show both acidic as well as basic behaviour. Such metal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides.
Detailed Explanation
This chunk explains that most metal oxides are basic, which means they can react with acids. However, some metal oxides exhibit amphoteric behavior, meaning they can react with both acids and bases. This duality allows them to produce salts and water, illustrating the versatility of certain metal oxides.
Examples & Analogies
Think of amphoteric oxides as bilingual friends who can speak two languages, allowing them to interact comfortably in various situations—whether it involves acids or bases, they can communicate effectively.
Reactivity Among Metals
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We have observed in Activity 3.9 that all metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen. Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
Detailed Explanation
This section discusses the variability in the reactivity of metals when exposed to oxygen. It highlights that while some metals, like potassium and sodium, are highly reactive and can catch fire, others are less reactive. This information is crucial for understanding how metals behave in different environments and the precautions needed for storage.
Examples & Analogies
Picture the metals as animals in a wild. Just as some animals are more aggressive or active than others, certain metals are 'wild' and react aggressively with their environment, necessitating careful handling to avoid accidents.
Protective Oxide Layers
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At ordinary temperature, the surfaces of metals such as magnesium, aluminium, zinc, lead, etc., are covered with a thin layer of oxide. The protective oxide layer prevents the metal from further oxidation. Iron does not burn on heating but iron filings burn vigorously when sprinkled in the flame of the burner. Copper does not burn, but the hot metal is coated with a black coloured layer of copper(II) oxide. Silver and gold do not react with oxygen even at high temperatures.
Detailed Explanation
This chunk explains the protective nature of oxide layers that form on certain metals, which can shield them against rapid oxidation. It also mentions that while some metals burn easily, others like iron and copper may not burn in bulk but can still oxidize when in fine forms or when heated. Notably, precious metals like silver and gold remain uninhibited by oxidation.
Examples & Analogies
Think of the oxide layer like a superhero shield protecting a city. Just as a shield can prevent destruction, the oxide layer guards the metal beneath, preserving it from further damage by air or moisture.
Anodising Process
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Anodising is a process of forming a thick oxide layer of aluminium. Aluminium develops a thin oxide layer when exposed to air. This aluminium oxide coat makes it resistant to further corrosion. The resistance can be improved further by making the oxide layer thicker. During anodising, a clean aluminium article is made the anode and is electrolysed with dilute sulphuric acid. The oxygen gas evolved at the anode reacts with aluminium to make a thicker protective oxide layer.
Detailed Explanation
Anodising involves electrochemically enhancing the protective oxide layer on aluminum. This thicker layer not only offers better resistance to corrosion but also allows for the possibility of dyeing it for aesthetic purposes. This process is important for extending the lifespan of aluminum products.
Examples & Analogies
Imagine coating an ice cream cone with chocolate to protect it from getting soggy. Just like that chocolate layer strengthens and preserves the ice cream, anodising strengthens aluminium against corrosion.
Conclusion on Reactivity
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
After performing Activity 3.9, you must have observed that sodium is the most reactive of the samples of metals taken here. The reaction of magnesium is less vigorous implying that it is not as reactive as sodium. But burning in oxygen does not help us to decide about the reactivity of zinc, iron, copper or lead. Let us see some more reactions to arrive at a conclusion about the order of reactivity of these metals.
Detailed Explanation
This conclusion emphasizes that direct observation of burning metals does not provide enough information to determine the complete reactivity series of metals. By indicating the need for further experiments, it encourages students to engage in active learning and exploration of chemical properties.
Examples & Analogies
Just like how you may need more than a single test to understand someone’s personality, determining a metal's reactivity requires a comprehensive study of various reactions to truly understand its character.
Key Concepts
-
Reactivity with Oxygen: Most metals react with oxygen when burned, forming metal oxides.
-
Metal Oxides: These compounds can be basic or amphoteric.
-
Protective Oxides: Some metals develop protective oxide layers that prevent further oxidation.
Examples & Applications
Magnesium burns with a bright white flame to form magnesium oxide.
Copper forms copper(II) oxide, a black oxide, upon burning.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the air, metals flare, turning bright, forming oxides in sight.
Stories
Once upon a time, metals gathered for a fiery showdown; magnesium shone bright, while copper stood still, untouched by the heat.
Memory Tools
Remember M.A.P. for Metal, Air to create Products (metal oxides).
Acronyms
O.A.R.
Oxides Are Reactive - a reminder of metal reactions with oxygen.
Flash Cards
Glossary
- Metal Oxide
A compound formed when a metal reacts with oxygen.
- Reactivity Series
A list that ranks metals based on their reactivity with other substances.
- Amphoteric Oxides
Metal oxides that can react with both acids and bases.
- Protective Layer
A thin layer of oxide that forms on some metals, preventing further oxidation.
Reference links
Supplementary resources to enhance your learning experience.
