Non-metals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Non-metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In our previous lessons, we discussed metals. Today, let’s shift our focus to non-metals. Can anyone share what they think non-metals are?

I think non-metals are the opposite of metals!

That’s correct! Non-metals indeed have properties that contrast with metals. They include elements like carbon, sulfur, and iodine. Now, who can tell me what characteristics make them unique?

I remember that non-metals do not conduct electricity well.

Exactly! Non-metals are poor conductors of electricity. Remember the acronym PONTS: Poor conductors, Often gases, Not malleable, Tend to form negative ions, and Solid or liquid states—this helps us remember their characteristics.
Physical Properties of Non-metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss physical properties. If we compare non-metals to metals, how do we think they differ in terms of hardness?

Non-metals are usually softer or brittle, right?

Good observation! Most non-metals are indeed brittle if they're solid. Now, how about their appearance?

I think they don't shine like metals do.

Correct! Non-metals lack metallic lustre. Now let’s move to conductivity. What can we say about non-metals in that aspect?

They don’t conduct heat or electricity well!

Exactly! Non-metals are bad conductors. Let’s summarize: Non-metals are typically soft, brittle, and non-conductive.
Reactivity and Chemical Properties of Non-metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the reactivity of non-metals. Who can give examples of non-metals and how they typically react?

Carbon, sulfur, and oxygen! I think they form acids.

Right! Non-metals often produce acidic oxides when combined with oxygen. Like sulfur forms sulfur dioxide, which is an acidic gas. How about non-metals and acids?

Non-metals don’t displace hydrogen from acids, unlike metals.

Exactly! That's a key difference. Let’s remember: Non-metals often form anions and tend to react with metals to form ionic compounds. Well done!
Comparison of Metals and Non-metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we’ve learned about non-metals, let’s compare them with metals. What are the fundamental differences?

Metals are good conductors, while non-metals are not!

Correct! And how about physical state at room temperature?

Most metals are solid, but some non-metals like oxygen are gases!

Very good! Remember the mnemonic MAD: Metals Are Ductile while non-metals are not. Great job recognizing these differences!

This helps a lot in understanding how we classify elements!

Exactly! And this classification is vital in chemistry!
Application of Non-metal Knowledge
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s wrap up today’s session by discussing the applications of non-metals. Can anyone provide examples of where we use non-metals in real life?

We use oxygen to breathe and carbon in food!

Exactly! And sulfur is used in fertilizers. What about iodine?

Iodine is used as a disinfectant!

Great examples! Non-metals may not be as abundant but they are vital. Remember, their unique properties allow us to use them in various essential applications. Well done today!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section delves into the characteristics of non-metals, highlighting their physical properties, examples, and the differences between them and metals. Non-metals such as carbon, sulfur, and iodine possess unique traits, including poor conductivity and lack of malleability and ductility.
Detailed
Non-metals
Non-metals make up a smaller group of elements compared to metals, and their characteristics significantly differ from those of metals. Key examples of non-metals include carbon, sulfur, iodine, oxygen, and hydrogen. Physical states of non-metals vary; most are either solids or gases, with bromine being a notable exception as a liquid.
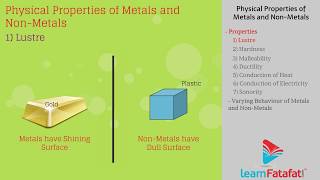
To categorize non-metals effectively, one must observe their physical properties in comparison to metals, as done through various activities involving carbon, sulfur, and iodine. During these activities, students can analyze the hardness, malleability, ductility, electrical conductivity, sonority, and physical appearance of both metals and non-metals, highlighting crucial differences.
Unlike metals, non-metals are generally non-lustrous, brittle (if solid), and they do not conduct electricity. They also form negatively charged ions, which contrasts with metals' tendency to form positive ions. The section emphasizes the significance of these properties in understanding the broader classification of elements and their chemical behaviors.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Non-metals
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the previous Class you have learnt that there are very few non-metals as compared to metals. Some of the examples of non-metals are carbon, sulphur, iodine, oxygen, hydrogen, etc. The non-metals are either solids or gases except bromine which is a liquid.
Detailed Explanation
This chunk introduces the concept of non-metals. Unlike metals, which are more abundant, non-metals are fewer in number. Examples provided include carbon, sulfur, iodine, oxygen, and hydrogen. It's notable that most non-metals are either solid or gaseous at room temperature, with bromine being an exception as it exists as a liquid.
Examples & Analogies
Think of non-metals like the quiet stars in the sky. There are fewer stars than there are grains of sand on a beach, but each star plays a vital role in the vast universe, just as non-metals are essential for life and various chemical processes.
Similarities with Metals
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Do non-metals also have physical properties similar to that of metals? Let us find out.
Detailed Explanation
In this chunk, the reader is prompted to consider whether non-metals possess physical properties akin to metals. This introductory question hints at the exploration of comparisons between the two classifications of elements, setting the stage for deeper investigations into their characteristics.
Examples & Analogies
It's like comparing apples and oranges: while both are fruits (like metals and non-metals are elements), they have distinctly different characteristics. This chunk invites us to learn what makes non-metals unique compared to metals.
Observing Non-metals through Activities
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Collect samples of carbon (coal or graphite), sulphur and iodine. Carry out the Activities 3.1 to 3.4 and 3.6 with these non-metals and record your observations.
Detailed Explanation
Here, students are instructed to gather samples of various non-metals such as carbon (available in forms like coal or graphite), sulfur, and iodine. The recommendation to conduct experiments encourages hands-on learning and observation, which is crucial for understanding the physical properties of non-metals.
Examples & Analogies
Think of it like a science chef experimenting with different ingredients. Just as a chef explores how each ingredient affects the taste and texture of a dish, students will explore how each non-metal behaves under similar conditions to uncover their properties.
Recording Observations
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Compile your observations regarding metals and non-metals in Table 3.1.
Detailed Explanation
In this step, students are encouraged to compile their observations concerning both metals and non-metals in a structured manner within a table. This organization helps facilitate comparison, making it easier to identify patterns and differences in properties such as hardness, malleability, ductility, conductivity, and sonority.
Examples & Analogies
Imagine creating a report card for metals and non-metals. Just as teachers assess students' performance in different subjects, compiling observations allows students to evaluate the 'performance' of these elements based on their physical properties.
General Properties of Non-metals
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
On the bases of the observations recorded in Table 3.1, discuss the general physical properties of metals and non-metals in the class. You must have concluded that we cannot group elements according to their physical properties alone, as there are many exceptions.
Detailed Explanation
This portion urges students to discuss their findings aloud, allowing for a collaborative learning experience. It emphasizes that physical properties alone are insufficient for classifying elements definitively, as notable exceptions exist within both non-metals and metals, inviting critical thinking.
Examples & Analogies
This is akin to the lesson that first impressions can be misleading. Just as a person might seem shy but is actually very outgoing, non-metals and metals may have surprising behaviors that extend beyond their initial physical traits.
Unique Characteristics of Non-metals
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For example – (i) All metals except mercury exist as solids at room temperature. In Activity 3.5, you have observed that metals have high melting points but gallium and caesium have very low melting points. (ii) Iodine is a non-metal but it is lustrous. (iii) Carbon is a non-metal that can exist in different forms.
Detailed Explanation
This chunk highlights various specific instances that illustrate the unique properties of non-metals. It mentions exceptions such as gallium and cesium, which challenge the typical notions of metal behavior. Furthermore, unique properties of iodine and carbon showcase how non-metals can exhibit characteristics that defy general expectations.
Examples & Analogies
Think of unique personalities in a group. While people may behave similarly in a group setting, individuals like iodine (lustrous) or carbon (existing in different forms like diamond and graphite) stand out, reminding us that each element has its own 'personality' that adds to the diversity of the periodic table.
Chemical Properties of Non-metals
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Most non-metals produce acidic oxides when dissolved in water. On the other hand, most metals give rise to basic oxides.
Detailed Explanation
This statement addresses the chemical behavior of non-metals, indicating their tendency to produce acidic oxides in contrast to metals that form basic oxides. Understanding the contrasting chemical properties is critical for recognizing how non-metals interact within chemical reactions.
Examples & Analogies
Imagine acidic lemonade versus basic soap. Each substance reacts differently when mixed with water. This analogy helps students grasp how non-metals alter their environment by forming acidic solutions through their chemical reactions.
Non-metals and Acids
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Non-metals do not displace hydrogen from dilute acids. They react with hydrogen to form hydrides.
Detailed Explanation
This chunk explains how non-metals interact with acids, emphasizing their inability to displace hydrogen, which contrasts with metals. Instead, non-metals often react with hydrogen to create compounds known as hydrides, underscoring their distinct chemical reactivity.
Examples & Analogies
Think of a dance where non-metals take a step toward hydrogen, creating a new dance called 'hydrides,' instead of trying to take the spotlight from hydrogen, which is what metals do by displacing it in acid reactions.
Key Concepts
-
Physical Properties of Non-metals: Generally non-lustrous, brittle, and poor conductors of heat and electricity.
-
Reactivity: Non-metals tend to form negative ions and can form acidic oxides when reacted with oxygen.
-
Classification: Non-metals are classified differently than metals; they lack properties such as malleability and ductility.
Examples & Applications
Examples of non-metals include carbon, sulfur, and oxygen. Carbon is crucial for organic molecules, sulfur is used in fertilizers, and oxygen is essential for respiration.
Common household items may contain non-metals, such as iodine in disinfectants and sulfur in matches.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Non-metals are not shiny, they don’t conduct well, they’re often found in air, and that’s easy to tell.
Stories
Imagine a party where metals are boasting about their strength and shine, but the non-metal friends quietly create beautiful songs, showing their importance without the need for glitz.
Memory Tools
Remember the acronym PONTS for non-metals: Poor conductors, Often gases, Not malleable, Tend to form negative ions, Solid or liquid.
Acronyms
Make yourself clear with NASTY
Non-metals Are Soft
Tend to yield negative ions.
Flash Cards
Glossary
- Nonmetal
Elements that are generally poor conductors of heat and electricity, brittle (if solid), and typically form negative ions.
- Malleability
The ability of a material to be deformed under compressive stress; metals are malleable while non-metals are not.
- Ductility
The ability of a material to be stretched into a wire; exhibited by metals but not by non-metals.
- Conductivity
The ability of a material to conduct electric current; non-metals are generally poor conductors.
- Sonority
The property of a material to produce a sound; metals usually exhibit sonority while non-metals do not.
Reference links
Supplementary resources to enhance your learning experience.
