Refining of Metals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Metal Refining
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore the importance of refining metals. Why do we need to refine metals in the first place?

To make them pure and suitable for use, right?

Exactly! Metals from reduction processes often have impurities. Can anyone name a commonly used refining method?

Is it electrolytic refining?

Correct! Electrolytic refining is the most prominent method used. Let's remember this with the acronym E.R. - Electrolytic Refining.

What happens in electrolytic refining?

Great question! The impure metal acts as the anode and pure metal is the cathode. This process separates the pure metal from impurities.

Where do the impurities go?

Some impurities dissolve in the electrolyte while insoluble ones settle as 'anode mud'. Remember this dynamic: E.R. for Electrolytic Refining!

To conclude, refining, especially electrolytic, is critical for ensuring the quality of metals. Remember E.R.!
Applications of Refined Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand how refining works, why do we care about the purity of metals in industries?

Purity probably affects how well they work in practical applications?

Absolutely! High purity equals better conductivity, strength, and durability. Think about copper wiring used in electronics.

So, is pure copper always better?

Yes, with increased purity comes efficiency in performance. This is why industries invest heavily in refining processes.

What other metals get refined?

We often refine zinc, silver, and gold too. Each purified metal has applications from construction to electronics!

In summary, refined metals ensure quality and efficiency across various industrial applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The refining process plays a crucial role in metallurgy, especially through electrolytic refining, which separates pure metals from impurities. This section highlights techniques for achieving high purity in metal product.
Detailed
Refining of Metals
The metals produced by various reduction processes described previously are not very pure as they contain several impurities that must be removed to acquire pure metals. One of the most widely used methods for refining impure metals is electrolytic refining.
Electrolytic Refining Process
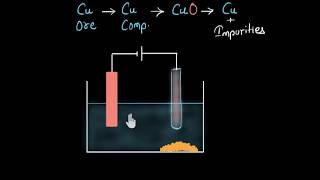
- Concept: In this process, the impure metal is adjusted as the anode while a thin strip of pure metal forms the cathode.
- Electrolyte: A solution of the metal salt serves as the electrolyte. For example, using acidified copper sulphate for copper refining.
- Process: As the current passes through the electrolyte, the pure metal from the anode dissolves into the solution. An equal amount of pure metal from the electrolyte deposits on the cathode. Any soluble impurities enter the solution, while insoluble impurities settle down at the bottom of the anode, forming what is known as anode mud.
This method is crucial for producing metals like copper, zinc, tin, nickel, silver, and gold in a pure state essential for various applications. The significance of this section lies in understanding how refining processes enhance the quality and utility of metals in industrial applications.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Metal Refining
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The metals produced by various reduction processes described above are not very pure. They contain impurities, which must be removed to obtain pure metals.
Detailed Explanation
The metals that we extract from ores are often contaminated with various impurities. This means that the metals are not in their pure form, which is essential for many applications. Therefore, refining is a necessary process that follows extraction. Refining helps to purify the metals, making them suitable for use in making various products. By removing these impurities, we can ensure that the metals have the desired properties and perform effectively in their respective applications.
Examples & Analogies
Think of refining as cleaning your clothes before wearing them. Just like dirt and stains can affect how good you look in an outfit, impurities in metals can affect their performance in real-world applications. If a metal isn't refined properly, it might not work effectively in electronics or construction, similar to how dirty clothes can make you feel uncomfortable or look unpresentable.
Electrolytic Refining Process
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The most widely used method for refining impure metals is electrolytic refining. Many metals, such as copper, zinc, tin, nickel, silver, gold, etc., are refined electrolytically.
Detailed Explanation
Electrolytic refining is a process that uses electricity to separate and purify metals. In this method, the impure metal is made the anode (the positive electrode) and a thin strip of pure metal serves as the cathode (the negative electrode). An electrolyte solution, usually a solution of a metal salt, is used to conduct electric current. When electric current passes, the impurities from the anode dissolve into the electrolyte, while pure metal from the solution gets deposited on the cathode. This process effectively separates pure metal from its impurities.
Examples & Analogies
Imagine baking a cake: you combine various ingredients to create a delicious dessert. In electrolytic refining, we start with a mixture of impurities (the 'cake batter') at the anode, and through the process of electrolysis, we create a pure metal cake at the cathode. Just as baking transforms raw ingredients into a finished cake, electrolytic refining transforms impure metal into high-quality metal.
Process Details of Electrolytic Refining
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The apparatus is set up in such a way that on passing the current through the electrolyte, the pure metal from the anode dissolves into the electrolyte. An equivalent amount of pure metal from the electrolyte is deposited on the cathode. The soluble impurities go into the solution, whereas, the insoluble impurities settle down at the bottom of the anode and are known as anode mud.
Detailed Explanation
In the electrolytic refining setup, the electric current facilitates the transfer of the pure metal from the anode into the electrolyte solution. As a result, the current causes the pure metal ions to migrate toward the cathode, where they deposit and form solid metal. Meanwhile, any soluble impurities also dissolve into the electrolyte solution, while insoluble impurities do not dissolve and fall to the bottom, creating anode mud. This separation of impurities enhances the quality of the extracted metal.
Examples & Analogies
Think of it like using a sieve to separate pasta from water after boiling. The pasta represents the pure metal that you want to keep, while the water symbolizes the electrolyte, capturing some of the particles (impurities) you don't want. The leftover pieces of food stuck in the sieve can be compared to the anode mud at the bottom that contains all the insoluble impurities you aimed to remove.
Key Concepts
-
Electrolytic Refining: A process to purify metals using an electrochemical method.
-
Anode Mud: Insoluble impurities that settle during refining.
-
Electrolyte: Solution used in the electrolysis that facilitates the purification process.
Examples & Applications
Copper refining uses copper sulfate as the electrolyte, where copper dissolves at the anode and pure copper forms at the cathode.
Zinc can also be purified through electrolytic refining, ensuring the removal of impurities.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
At the anode, metals dissolve, on the cathode, pure resolves.
Stories
Imagine a crowded party (the impurities) where some friends leave the room (anode mud), leaving behind the real party-goers (pure metal) at the center of fun (cathode).
Memory Tools
Remember: 'C.A.A.' for Copper Anode Analyzing - where copper is analyzed for purity!
Acronyms
E.R. stands for Electrolytic Refining, highlighting its core purpose.
Flash Cards
Glossary
- Electrolytic Refining
A method of purifying metals by using an electrolytic cell where the metal dissolves and deposits on the cathode.
- Anode
The positively charged electrode in an electrolytic cell where oxidation occurs.
- Cathode
The negatively charged electrode in an electrolytic cell where reduction occurs.
- Electrolyte
A solution containing ions that conducts electricity and is used in the electrolytic refining process.
- Anode Mud
The inert impurities that settle at the bottom of the electrolytic cell during refining.
Reference links
Supplementary resources to enhance your learning experience.
