Physical Properties
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning class! Today, we are going to explore the physical properties of metals. Can anyone tell me what you notice about metals when you see them?

They're shiny!

Exactly! This shine is called metallic lustre. Metals like iron and copper have this property. Now, can someone tell me how many metals you know that are used in your homes?

I think aluminum is one. We use it for cooking pans.

Great example! Aluminum is not only lustrous but also malleable, meaning it can be beaten into thin sheets without breaking. Let's remember that with the acronym 'LMA' – Lustrous, Malleable, and Ductile. Can anyone give a definition for ductile?

Ductility means it can be drawn into wires, right?

Perfect! Metals have the ability to be stretched into wires. Let’s summarize today: metals are shining, malleable, ductile, and generally good conductors of heat and electricity.
Physical Properties of Non-Metals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've explored metals, let’s move on to non-metals. What can you tell me about them?

They aren't shiny like metals.

Correct! Non-metals lack the metallic lustre. Besides that, they are generally brittle and poor conductors of heat and electricity. Can you name any common non-metals?

Carbon and sulfur are two examples!

That's right! Carbon can come in different forms, such as diamond and graphite, each with unique properties. Memory aids are helpful! Can we use an acronym for non-metals? Let's say 'BNPS': Brittle, No Lustre, Poor Conductors, and Solid or Gaseous state. What do you think?

Sounds great! It’s easy to remember.

Fantastic! So, non-metals are not only different from metals in appearance but also in their physical properties.
Conductivity and Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

One significant property of metals is their conductivity. Can anyone explain what we mean by conductivity and where we encounter it?

It means metals can carry electrical current! We see it in wires.

Exactly! Metals like copper are extensively used for wiring because they conduct electricity well. Remember, non-metals like sulfur do not conduct electricity. Think of the 'SLED' acronym for non-metals: Sulfur is Less Electrical conductor, and Density differs as well. Why is understanding these properties important?

Because it helps us know what materials to use for different applications!

Well said! Knowing the properties helps in selecting the right materials for everyday uses, from cooking utensils to electrical components.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The exploration of physical properties in this section categorizes metals and non-metals based on their intrinsic characteristics. Through various activities, students learn how metals exhibit lustre, malleability, ductility, conductivity, and sonority while contrasting these with non-metals, illustrating their distinct behaviors and applications in everyday life.
Detailed
Physical Properties
This section delves into the classification of elements as metals and non-metals based on their physical properties. In Class IX, students learned about different elements' roles and their properties, setting the stage for exploring their uses and significance. The key physical properties explored include:
- Metals:
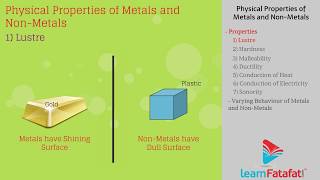
- Lustre: Metals like iron, copper, and aluminum have a shiny appearance resulting from their metallic lustre.
- Hardness: Most metals are hard, although their hardness varies (e.g., sodium is soft and can be cut easily).
- Malleability: Metals can be beaten into thin sheets, a property particularly evident in metals like gold and silver.
- Ductility: Metals can be drawn into wires, with gold being the most ductile.
- Conductivity: Metals are good conductors of heat and electricity, as demonstrated by activities involving heating and electric circuits.
- Sonority: Metals produce a sound when struck, which is why they are used in instruments like bells.
- Non-metals:
- In contrast, non-metals are usually brittle, poor conductors, and can exist in solid, liquid, or gaseous states. For example, carbon, sulfur, oxygen, and hydrogen are common non-metals, each exhibiting unique physical properties that determine their use and applications.
Overall, this section emphasizes the contrasting physical characteristics of metals and non-metals, discussing their implications in everyday applications and industrial uses.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Physical Properties of Metals
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The easiest way to start grouping substances is by comparing their physical properties. Let us study this with the help of the following activities. For performing Activities 3.1 to 3.6, collect the samples of the following metals – iron, copper, aluminium, magnesium, sodium, lead, zinc and any other metal that is easily available.
Detailed Explanation
In this section, we begin with the classification of substances into metals and non-metals based on their physical properties. Physical properties include characteristics that can be observed or measured without altering the substance, such as appearance, hardness, malleability, ductility, and conductivity. We will conduct a series of activities with different metals to explore these properties.
Examples & Analogies
Consider a set of tools in a toolbox—hammers, screwdrivers, and pliers. Each tool is made of different metals, and their physical properties determine their function. For instance, hammers need to be hard and able to withstand force, while screwdrivers need to be firm yet can be slender to fit into tight spaces.
Metallic Lustre
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Metals, in their pure state, have a shining surface. This property is called metallic lustre.
Detailed Explanation
Metallic lustre refers to the shiny appearance of metals when they are cut or polished. This lustre is due to the ability of metal atoms to absorb and re-emit light. When you clean or rub a metal surface, the scratches that could dull it are minimized, resulting in a more reflective surface, further demonstrating this property.
Examples & Analogies
Think of a brand new coin or a piece of jewelry. The shiny surface you observe is a result of metallic lustre. Just like how polishing your shoes makes them shine, cleaning metal surfaces enhances their lustrous appearance.
Hardness of Metals
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You will find that metals are generally hard. The hardness varies from metal to metal.
Detailed Explanation
The hardness of metals is an important physical property and varies across different metals. For example, iron is significantly harder compared to aluminum. This aspect of metals plays a critical role in determining their use in construction and manufacturing. Generally, harder metals can withstand more stress and are suitable for heavy-duty applications.
Examples & Analogies
Consider how different tools are made from different metals. A knife needs to be sharp and hard to cut through materials effectively, while a frying pan made of softer metal can be easily shaped and heated to cook food.
Malleability of Metals
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You will find that some metals can be beaten into thin sheets. This property is called malleability. Did you know that gold and silver are the most malleable metals?
Detailed Explanation
Malleability is the ability of a metal to be hammered or rolled into thin sheets without breaking. This property allows metals to be shaped for various applications. Gold and silver, due to their exceptional malleability, can be crafted into intricate designs using very thin sheets that are still strong.
Examples & Analogies
Imagine working with a piece of clay. When you flatten it out with a rolling pin, it becomes a thin sheet. Similarly, metals like gold can be pounded into delicate, thin sheets known as gold leaf that can be used for decorative purposes.
Ductility of Metals
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ability of metals to be drawn into thin wires is called ductility. Gold is the most ductile metal.
Detailed Explanation
Ductility is the property that allows metals to be stretched into wires without breaking. Gold is particularly known for this property; a single gram can be drawn into a wire over 2 km long due to its flexibility and strength. This property is crucial for electrical wiring and various applications where flexibility is needed.
Examples & Analogies
Think of a rubber band. When you stretch it, it can become much longer without breaking. Similarly, metals like gold can be drawn out into long wires, which are used extensively in electronics and jewelry.
Conductivity of Metals
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Metals are good conductors of heat and have high melting points. The best conductors of heat are silver and copper.
Detailed Explanation
Metals are primarily known to conduct heat and electricity efficiently. This is due to the presence of free-moving electrons within metal structures, allowing them to transfer heat or electrical energy rapidly. Silver is the best conductor of heat, followed closely by copper, which is widely used in electrical wiring.
Examples & Analogies
If you've ever held a metal spoon in a pot of hot soup, you'll notice the spoon gets hot quickly. This is because metals conduct heat, transferring the warmth from the soup to your hand efficiently.
Sonority of Metals
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The metals that produce sound on striking a hard surface are said to be sonorous. Can you now say why school bells are made of metals?
Detailed Explanation
Sonority refers to the property of metals to produce a ringing sound when struck. This quality is particularly beneficial for musical instruments and, notably, for bells that need to produce clear sound. The characteristic is due to the stiffness and elasticity of the metal used.
Examples & Analogies
Think of the sound a bell makes when you hit it. The metal resonates, producing a clear tone that carries well. This is why bells are often made of metals like bronze or brass, which are sonorous.
Key Concepts
-
Lustre: The shiny appearance of metals.
-
Malleability: Metals can be hammered or rolled into thin sheets.
-
Ductility: Metals can be drawn into wires.
-
Conductivity: Metals are good conductors of electricity and heat.
-
Sonority: Metals produce a sound when struck.
-
Non-metals: Typically lack metallic properties and are poor conductors.
Examples & Applications
Gold and silver are examples of highly malleable metals, used in jewelry and decorative items.
Aluminum is a ductile metal used in electrical wiring and manufacturing of cans and foils.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Metals shine bright, ductile and tough, malleable too, for forms they are rough.
Stories
Imagine a metallurgist in a castle, crafting metal to make a sword. The metal shines and stretches as they hammer, creating a beautiful weapon that is also strong.
Memory Tools
LDMCS: Lustrous, Ductile, Malleable, Conductive, Sonorous - remember the properties of metals!
Acronyms
Noble each non-metal is
Non-conductive
Opaque
Brittle
Less sonorous
Electrical insulator.
Flash Cards
Glossary
- Lustre
The shiny appearance of a metal.
- Malleability
The ability of a metal to be hammered or rolled into thin sheets.
- Ductility
The ability of a metal to be drawn into wires.
- Conductivity
The ability of a material to conduct electricity or heat.
- Sonority
The quality of a substance to produce a sound when struck.
- Nonmetals
Elements that typically lack metallic luster and are poor conductors of heat and electricity.
Reference links
Supplementary resources to enhance your learning experience.
