How do Metals react with Solutions of other Metal Salts?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Displacement Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss how metals react with solutions of other metal salts. Specifically, we will explore what happens during displacement reactions. Can anyone tell me what a displacement reaction is?

Isn't it when one metal displaces another from its salt solution?

Exactly! In a displacement reaction, a more reactive metal can displace a less reactive metal from its salt solution. For example, if we have copper in a solution of iron sulfate, what might happen?

The copper will not react because it's less reactive than iron.

Correct! That’s the crux of understanding reactivity among metals. Let's summarize: Displacement reactions help us identify the order of reactivity in metals.
Conducting a Classroom Experiment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s perform a practical experiment. We will use copper wire and an iron nail. First, we will place the copper wire in iron sulfate and record our observations after 20 minutes. What do you think we are looking for?

We should see if there's any change in the solution or the metals.

Exactly! After that, we will switch positions and put the iron nail in a copper sulfate solution. Let's analyze what happens.

I'm excited to see if the iron will replace the copper in the solution.

Very good! Always remember, iron is more reactive than copper. So, if a displacement reaction occurs, iron will take copper's place in the solution.
Analyzing Observations and Results
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What did you all observe after our experiments? Did we see a change?

The copper wire did not change, but the iron nail in the copper sulfate turned reddish-brown.

And the solution turned colorless after we placed the nail in it.

Exactly! The reddish-brown color indicates copper has been displaced. Write down the balanced equation for this reaction as a group.

Is it Fe + CuSO₄ → Cu + FeSO₄?

Correct! Remember, identifying these reactions helps us create a reactivity series for metals.
Concept of Reactivity Series
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s take a moment to discuss the reactivity series. Can anyone tell me why it matters?

It shows how different metals react with each other and with compounds, right?

Exactly! The reactivity series allows us to predict which metals will react with certain compounds. For example, sodium and potassium are very reactive, while metals like gold and silver are much less reactive.

So, if a metal is high on the reactivity series, it will always displace the metals lower than it?

That's correct! Always remember: The more reactive a metal, the more likely it is to displace a less reactive metal from its salt solution.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details the displacement reactions between metals and their effects on metal salt solutions. It emphasizes the concept of reactivity, using copper and iron as examples to illustrate how more reactive metals displace less reactive metals. This leads to a broader discussion on the reactivity series of metals.
Detailed
In the context of chemical reactions, the section investigates how certain metals respond when placed in solutions of metal salts, specifically focusing on displacement reactions. When a more reactive metal is introduced to a salt solution containing a less reactive metal, a reaction occurs where the less reactive metal is displaced. For example, when copper wire is placed in an iron(II) sulfate solution and an iron nail is submerged in a copper(II) sulfate solution, observable reactions occur that provide insights into the reactivity series of metals. This section not only highlights individual reactions but also conceptualizes the broader framework of metal reactivity, which aids in predicting the outcomes of various metal interactions. A balanced chemical equation can be written for the reactions observed, reinforcing the understanding of displacement reactions.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Observing Displacement Reactions
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
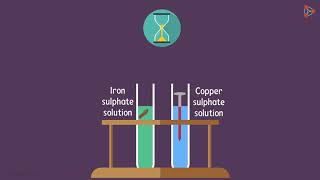
n Take a clean wire of copper and an iron nail.
n Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in test tubes (Fig. 3.4).
n Record your observations after 20 minutes.
n In which test tube did you find that a reaction has occurred?
n On what basis can you say that a reaction has actually taken place?
n Can you correlate your observations for the Activities 3.9, 3.10 and 3.11?
n Write a balanced chemical equation for the reaction that has taken place.
n Name the type of reaction.
Detailed Explanation
This chunk describes an activity involving displacement reactions between metals and their salt solutions. In this experiment, students will examine how copper and iron react when placed in solutions of one another’s salts. By observing changes in the appearance of the solutions and the metals, students can determine if a reaction occurred. If a metal changes color or if a solid (precipitate) forms, these indicate a reaction. A balanced chemical equation must then be written to describe the reaction quantitatively, identifying the products formed.
Examples & Analogies
Think of this reaction like a game of musical chairs. If a more popular kid (a more reactive metal) comes into the game, they can take the chair (displace the other metal) from the less popular kid. So, in this case, if copper can 'take' the position of iron in its salt solution, it indicates that copper is less reactive compared to iron.
Understanding Reactivity in Metals
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
We have seen in the previous sections that all metals are not equally reactive. We checked the reactivity of various metals with oxygen, water and acids. But all metals do not react with these reagents.
So we were not able to put all the metal samples we had collected in decreasing order of their reactivity. Displacement reactions studied in Chapter 1 give better evidence about the reactivity of metals. It is simple and easy: if metal A displaces metal B from its solution, it is more reactive than B.
Reaction of metals with salt solutions:
Metal A + Salt solution of B → Salt solution of A + Metal B.
Detailed Explanation
This chunk explains the concept of displacement reactions in metals. It notes that reactive metals will displace less reactive metals from their salt solutions. The reactivity series provides a guide on how to predict which metals will displace others. If a metal in a reaction can successfully replace another from its solution, it proves that it is more reactive than the metal being replaced.
Examples & Analogies
Imagine a classroom where a popular student can easily take a place next to the teacher (the more reactive metal can displace the less reactive one). Similarly, in a running race, if competitor A can outpace competitor B, they are faster (more reactive) – just like in metals, if A can displace B from a salt solution, A is considered more reactive.
Identifying Reactivity through Experimentation
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Which metal, copper or iron, is more reactive according to your observations in Activity 3.12?
Detailed Explanation
This question encourages students to analyze their previous experimental results from the activities done. By observing which metal displaced the other, students can conclude which is more reactive. If iron displaces copper from the copper sulfate solution, iron is more reactive. Conversely, if copper did not displace iron from the iron sulfate solution, this helps cement understanding of the pattern of reactivity.
Examples & Analogies
Consider a story where a small dog challenges a larger dog to a bark-off. If the larger dog backs down, the smaller dog is deemed 'braver,' just like the reactive metal that takes over a salt solution. This experiment helps students associate real-life decision-making with the concept of reactivity.
Key Concepts
-
Displacement Reaction: A more reactive metal can displace a less reactive metal from its salt solution.
-
Reactivity Series: Metals are arranged based on their ability to react, allowing predictions about displacement.
-
Balanced Chemical Equation: Helps in understanding the reactants and products of reactions.
Examples & Applications
When iron is placed in a copper sulfate solution, it displaces copper, forming iron sulfate and solid copper.
Copper does not react with iron sulfate, demonstrating its lower reactivity compared to iron.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In displacement reactions, metals play, / The strong will push the weak away.
Stories
Once there was iron, so bold and brave, it met copper in a salt wave. Iron pushed copper out of play, and that’s how metals find their way.
Memory Tools
Remember: 'Iron beats Copper' (IBC) for displacement.
Acronyms
Use RANK
Reactivity Arranges Noble Kin (to remember the order of reactivity) between metals.
Flash Cards
Glossary
- Displacement Reaction
A chemical reaction where a more reactive metal displaces a less reactive one from its compound.
- Reactivity Series
A list of metals arranged in order of decreasing reactivity.
- Salt Solution
A solution formed when a salt is dissolved in water.
Reference links
Supplementary resources to enhance your learning experience.
