Formation of Ionic Compounds
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ionic Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

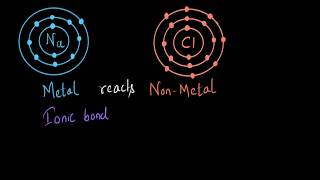
Today, we're going to learn about ionic compounds! Can anyone tell me what happens when a metal and a non-metal react together?

I think the metal loses electrons to the non-metal.

Exactly! When metals lose electrons, they become positively charged ions called cations. What happens to the non-metal?

The non-metal gains those electrons and becomes negatively charged!

Great! These oppositely charged ions attract each other and form an ionic bond. This makes compounds like sodium chloride. Does anyone remember what sodium becomes when it loses an electron?

Sodium becomes Na+!

Correct! So, sodium gives away one electron to become Na+, and what about chlorine?

Chlorine becomes Cl- after gaining that electron!

Exactly! So the formula for sodium chloride is NaCl. In summary, ionic compounds form by the transfer of electrons from metals to non-metals.
Properties of Ionic Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've covered how ionic compounds form, can anyone list some properties of these compounds?

They have high melting and boiling points!

That's correct! The strong electrostatic forces between ions require a lot of energy to break. What about their solubility?

They usually dissolve in water!

Exactly! Ionic compounds like sodium chloride dissolve easily in water due to the attraction between the ions and water molecules. But how do they conduct electricity?

They conduct electricity when dissolved in water or melted!

Right! In solid form, ionic compounds don’t conduct electricity, but when they are dissolved or melted, the ions are free to move and carry the electric charge. Summing up, ionic compounds are typically solid, high-melting, soluble in water, and good conductors in liquid states.
Formation Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's analyze some common examples of ionic compounds. What do you think is formed when magnesium reacts with chlorine?

Magnesium chloride, right?

Exactly! Magnesium loses two electrons to form Mg2+. How about sulfur?

It gains two electrons to form S2-.

Correct! So, when we combine Mg2+ and S2-, we get magnesium sulfide. This illustrates the concept of ionic compounds very well. Each of these forms an essential part of our daily life.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The formation of ionic compounds occurs when metals lose electrons to become positively charged cations, while non-metals gain those electrons to form negatively charged anions. This section discusses the process of electron transfer and introduces key ionic compounds formed through this process.
Detailed
Formation of Ionic Compounds
In this section, we explore how ionic compounds are formed through the transfer of electrons between metals and non-metals. Metals, which have a tendency to lose electrons, form cations, while non-metals gain these electrons to form anions. The electrostatic attraction between these oppositely charged ions produces ionic bonds, resulting in the formation of ionic compounds such as sodium chloride (NaCl) and magnesium chloride (MgCl2).
Key Points:
- Electron Transfer: Metals lose electrons to form positive ions, and non-metals gain them to form negative ions.
- General Equation:
- Parametrically, the reaction can be illustrated as:
Metal → Metal^n+ + ne^-(for metals)
Non-metal + n(e^-) → Non-metal^-n(for non-metals) - Examples: Sodium chloride (NaCl) and magnesium chloride (MgCl2) demonstrate the ionic bond formation through the transfer of electrons.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Electron Transfer in Ionic Compounds
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The compounds formed in this manner by the transfer of electrons from a metal to a non-metal are known as ionic compounds or electrovalent compounds.
Detailed Explanation
Ionic compounds are created through the transfer of electrons. When a metal, which typically has a few electrons in its outer shell, loses one or more electrons, it becomes positively charged. Conversely, a non-metal which usually has more electrons in its outer shell gains those electrons and becomes negatively charged. This transfer of electrons results in the formation of ions: cations (positively charged) from metals and anions (negatively charged) from non-metals. The opposite charges of these ions create strong electrostatic forces of attraction, which hold the compound together.
Examples & Analogies
Imagine a pair of friends at a party: one is extroverted and loves to share (the metal) while the other is introverted and loves to gather things (the non-metal). The extroverted friend gives away their phone (an electron) to the introverted friend, who is thrilled to receive it because it completes their collection of gadgets. Now, they are bonded together as best friends, just like how cations and anions bond to form ionic compounds.
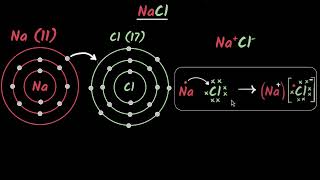
Formation of Sodium Chloride
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Na → Na++e– (Sodium cation)
Cl +e–→Cl– (Chloride anion)
Detailed Explanation
The formation of sodium chloride (table salt) illustrates the creation of an ionic compound. When sodium (Na) reacts with chlorine (Cl), sodium loses one electron to become a sodium ion (Na⁺), while chlorine gains that electron to become a chloride ion (Cl⁻). This electron transfer is essential because it allows both elements to achieve a more stable electron configuration, similar to that of noble gases. As a result, Na⁺ and Cl⁻ are attracted to each other due to their opposite charges, forming sodium chloride (NaCl).
Examples & Analogies
Think of sodium and chlorine as two puzzle pieces: when you have one piece with a protruding part (sodium) that fits into a slot (chlorine), a complete picture is formed when they connect. Just like the pieces fit together perfectly to complete a puzzle, sodium and chloride ions join to create the stable structure of sodium chloride.
Formation of Magnesium Chloride
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Mg→Mg2++2e– (Magnesium cation)
Cl +e– → Cl– (Chloride anion)
Detailed Explanation
The process of forming magnesium chloride (MgCl₂) works similarly to sodium chloride. Here, magnesium (Mg) loses two electrons, becoming a magnesium ion (Mg²⁺), while each chlorine atom gains one electron to become chloride ions (Cl⁻). Two chloride ions are needed to balance the +2 charge of the magnesium ion, resulting in the compound MgCl₂. This is a useful example of how the charges of ions determine the formula of ionic compounds, ensuring the total positive and negative charges cancel each other out.
Examples & Analogies
It's like a team sports scenario where one player (magnesium) needs two teammates (chloride ions) to balance out their stronger position. For victory, the team has to stay equal; hence, for every one magnesium ion, two chloride ions are required, just like how MgCl₂ balances the charges to maintain stability in an ionic compound.
Properties of Ionic Compounds
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ionic compounds have high melting and boiling points... These compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
Detailed Explanation
Ionic compounds exhibit distinct properties due to their ionic nature. They are generally solids at room temperature, known for their hardness and brittleness because the ions are held together by strong electrostatic forces. These compounds have high melting and boiling points, as significant energy is required to break the bonds between the ions. Additionally, ionic compounds tend to dissolve in water because water molecules can surround and separate the ions, but they do not dissolve in organic solvents like kerosene or petrol due to the lack of polar interactions in those solvents.
Examples & Analogies
Consider ionic compounds like a tightly packed group of people at a concert. They're difficult to disrupt (high melting/boiling points), and if someone tries to separate them (applying heat), it requires a lot of effort. However, when you pour water (like the energy of a concert-goer stepping in with a megaphone) on them, they easily disperse as the water splits them apart and carries them away.
Electrical Conductivity of Ionic Compounds
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The conduction of electricity through a solution involves the movement of charged particles.
Detailed Explanation
Ionic compounds can conduct electricity when they are dissolved in water or molten. This happens because, in solutions, the ionic compounds dissociate into their constituent ions, which are free to move and carry electric current. However, as solids, ionic compounds cannot conduct electricity because the ions are fixed in place within a rigid lattice structure. This distinction is crucial for understanding how and why ionic compounds can be used in electrochemical applications.
Examples & Analogies
Think of the solid ionic compound like a house with the doors locked (no conduction in solid state). When you dissolve the compound in water and open the doors, it's like letting people (ions) flow freely. Just as a crowd can move and reach different spots when doors open, ions can move to conduct electricity when the compound is dissolved or melted.
Key Concepts
-
Ionic Bond: A bond formed between cations and anions due to electrostatic attraction.
-
Electronegativity: The tendency of an atom to attract electrons, leading to ionic bonding.
Examples & Applications
Sodium Chloride (NaCl): Formed from Na+ and Cl- ions.
Magnesium Chloride (MgCl2): Formed from Mg2+ and Cl- ions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Cation gives, anion takes, ionic bond, strong it makes!
Stories
Imagine a knight (metal) giving away his sword (electron) to a defender (non-metal), who becomes stronger (anion) by keeping it safe.
Memory Tools
C-A-I (Cations are Always Ions) to remember what ions are formed from metals.
Acronyms
I.C.E. (Ion, Charge, Electron) - Ionic compounds involve ions, have charges, and involve electron transfer.
Flash Cards
Glossary
- Ionic Compound
A compound formed by the electrostatic attraction between positively and negatively charged ions.
- Cation
A positively charged ion that is formed when an atom loses electrons.
- Anion
A negatively charged ion that is formed when an atom gains electrons.
- Electrostatic Attraction
The force of attraction between opposite charges, such as cations and anions.
Reference links
Supplementary resources to enhance your learning experience.
