Biomacromolecules
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Biomacromolecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into biomacromolecules. Can anyone tell me what biomacromolecules are?

Are they just large molecules in our body?

Correct! They are large organic compounds such as proteins, nucleic acids, polysaccharides, and lipids. Now, how do we classify them?

By their size, right? Like macromolecules versus micromolecules.

Exactly! Micromolecules are smaller than a thousand daltons, while biomacromolecules have molecular weights of at least ten thousand daltons. Remember the acronym **LPNP** for Lipids, Proteins, Nucleic acids, and Polysaccharides!

What about their roles?

Great question! Biomacromolecules are essential for structure and function in living organisms. They contribute to processes like digestion, energy storage, and DNA replication.

So they are like building blocks?

Precisely! They are the building blocks of life. Let's sum it up: biomacromolecules are critical for all biological processes.
Composition of Biomacromolecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss the elemental composition of cells. What is the most abundant component?

Water, right?

Correct! Water makes up 70-90% of the cellular mass. Can anyone name the major biomacromolecules and their approximate contents?

Proteins and nucleic acids?

Yes, proteins constitute about 10-15% and nucleic acids 5-7%. Importantly, lipids, though often found in smaller quantities, also play significant structural roles in cells.

Is there a specific order for these components?

Absolutely! Here’s a mnemonic: **PWNCL** - Protein, Water, Nucleic acids, Carbohydrates, and Lipids. Knowing the percentage composition helps understand cell functions!

That makes it easier to remember!

To summarize, the key players in cellular composition are all biomacromolecules with unique roles in maintaining life.
Understanding Lipids as Biomacromolecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s tackle the question of lipids. Why do you think lipids are considered macromolecules?

Because they make up cell membranes?

Exactly! While individual lipids may be smaller than 800 daltons, they aggregate to form essential structures like membranes. Can anyone explain why this distinction is important?

It highlights how their function, not just their size, is important!

Correct! This idea reinforces that size isn’t everything. Remember that lipids play critical roles in energy storage and membrane formation!

So, they have collective properties?

Very well said! Let’s wrap up this session: lipids, though small individually, create vast impacts when working together in biological systems.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Biomacromolecules represent a significant class of organic compounds and include proteins, nucleic acids, polysaccharides, and lipids, distinguished by their larger molecular weights. These compounds are vital for various biological functions and structures in living tissues.
Detailed
Biomacromolecules
Biomacromolecules are large organic compounds that include proteins, nucleic acids, polysaccharides, and lipids. They are typically characterized by their high molecular weights, often exceeding ten thousand daltons. These biomolecules play essential roles in the structure and function of living organisms.
- Definition and Classification: Biomacromolecules consist of both organic and inorganic compounds found in living organisms, chiefly proteins, nucleic acids, polysaccharides, and lipids.
- Distinction from Micromolecules: Micromolecules are smaller biomolecules with molecular weights of less than a thousand daltons, while biomacromolecules are typically larger, with significant functional roles in cellular processes.
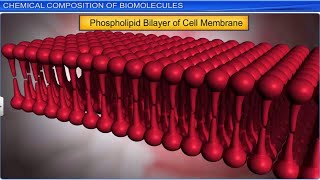
- Lipids: Although classified typically under macromolecules, lipids, which are usually smaller than 800 daltons, are included in the acid-insoluble fraction due to their aggregation into structures like cell membranes, contributing to the discrepancy in classification.
- Chemical Composition: The primary components of cells include water (70-90%), proteins (10-15%), carbohydrates (3%), and lipids (2%), with nucleic acids constituting 5-7% of cellular mass.
- Significance: Understanding biomacromolecules is crucial for grasping the molecular biology of life forms and their metabolic pathways, emphasizing their roles in cell structure and functionality.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Types of Biomolecules
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
There is one feature common to all those compounds found in the acid soluble pool. They have molecular weights ranging from 18 to around 800 daltons (Da) approximately.
The acid insoluble fraction, has only four types of organic compounds i.e., proteins, nucleic acids, polysaccharides and lipids. These classes of compounds with the exception of lipids, have molecular weights in the range of ten thousand daltons and above.
Detailed Explanation
In living organisms, biomolecules can be categorized based on their molecular weights. Compounds that are soluble in acid typically have lower molecular weights, ranging from 18 to about 800 daltons. In contrast, biomolecules that are insoluble in acid, such as proteins, nucleic acids, polysaccharides, and lipids, usually have much higher molecular weights, typically exceeding ten thousand daltons.
This distinction is important because it separates smaller, water-soluble compounds (like sugars and amino acids) from larger, structural forms like proteins and nucleic acids, which play crucial roles in the structure and function of the organisms.
Examples & Analogies
Think of this like comparing small LEGO blocks to large LEGO structures. The smaller blocks represent micromolecules that easily dissolve in water, while the complex structures represent macromolecules that can provide structure and function in a larger piece, similar to how proteins and nucleic acids work in living cells.
Biomacromolecules Classification
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For this very reason, biomolecules, i.e., chemical compounds found in living organisms are of two types. One, those which have molecular weights less than one thousand dalton and are usually referred to as micromolecules or simply biomolecules while those which are found in the acid insoluble fraction are called macromolecules or biomacromolecules.
Detailed Explanation
Biomolecules can be classified into two distinct categories based on their size and solubility. Micromolecules, or simply biomolecules, are small compounds with molecular weights less than one thousand daltons. These include substances like sugars and amino acids, which are crucial for various metabolic processes. On the other hand, macromolecules, known as biomacromolecules, are larger, complex structures found in the acid-insoluble fraction, such as proteins, nucleic acids, polysaccharides, and to some extent lipids, despite their smaller size. This classification helps in understanding the different roles these molecules play in the biology of organisms.
Examples & Analogies
This is similar to categorizing tools based on their usage: small tools like screwdrivers and wrenches are like micromolecules that help with fine tasks, while larger tools like drills and saws are akin to macromolecules, which are used for heavier, structural work.
Role of Lipids in Biomacromolecules
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Then why do lipids, whose molecular weights do not exceed 800 Da, come under acid insoluble fraction, i.e., macromolecular fraction? Lipids are indeed small molecular weight compounds and are present not only as such but also arranged into structures like cell membrane and other membranes.
Detailed Explanation
Although lipids have molecular weights that typically do not exceed 800 daltons, they are classified under macromolecules because they play a significant structural role in cells, particularly in forming membranes. When biological tissues are broken down, the lipid molecules are not just free; they are often found as part of larger structures like cell membranes. This unique property of lipids makes them part of the macromolecule category, even though individually they are smaller than typical macromolecules like proteins.
Examples & Analogies
Imagine a balloon filled with air. The air inside (lipids) is lightweight and could be considered small, but when the balloon is formed (the cell membrane), it becomes significant and large, showing how small components can create essential structures.
Chemical Composition and Abundance
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The acid soluble pool represents roughly the cytoplasmic composition. The macromolecules from cytoplasm and organelles become the acid insoluble fraction. Together they represent the entire chemical composition of living tissues or organisms.
Detailed Explanation
The acid-soluble pool contains various small molecules that are typically found in the cytoplasm of cells, while the acid-insoluble fraction consists of large macromolecules derived from the cytoplasm and organelles. The combination of these two fractions provides a comprehensive picture of the chemical makeup of living tissues, reflecting an organism’s molecular diversity, critical for its structure and function.
Examples & Analogies
Think of this like making a smoothie. The liquid part, which includes fruits and other nutrients, is like the acid-soluble fraction, providing immediate energy, while the chunks of fruit and other solid ingredients represent the macromolecules that give fiber and longer-lasting energy.
Abundance of Key Components in Living Organisms
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In summary if we represent the chemical composition of living tissue from abundance point of view and arrange them class-wise, we observe that water is the most abundant chemical in living organisms.
Detailed Explanation
When looking at the composition of living tissues, water is the most abundant chemical component. It serves various essential roles, from being a solvent for biochemical reactions to helping maintain cell shape and temperature regulation. Following water, other important biomolecules like proteins, carbohydrates, lipids, and nucleic acids exist in smaller amounts but are vital for life processes.
Examples & Analogies
Consider water as the foundation of a house; without it, the rest of the structure wouldn’t stand. Just as a house needs a solid foundation for support and stability, living organisms need water for their biological integrity and functioning.
Key Concepts
-
Molecular Weight: Biomacromolecules are typically over 10,000 daltons.
-
Types of Biomacromolecules: Major types include proteins, nucleic acids, polysaccharides, and lipids.
-
Lipids Role: Though low molecular weight, lipids are crucial for membrane structure.
Examples & Applications
Proteins like enzymes that catalyze biochemical reactions.
Lipids form cell membranes and store energy.
Polysaccharides like starch and glycogen serve as energy reserves.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Lipids, proteins, and nucleic acids too, polysaccharides in cells, that's what they do!
Stories
Once in a cell, there were proteins, lipids, and more. They all worked together to open cellular doors.
Memory Tools
Remember PLNP for Proteins, Lipids, Nucleic Acids, and Polysaccharides.
Acronyms
Use **BLOPS** for Biomacromolecules
Big Lipids
Outstanding Proteins
Legendary Nucleic Acids
Pleasurable Polysaccharides
Significantly Important.
Flash Cards
Glossary
- Biomacromolecules
Large organic compounds in living organisms, including proteins, nucleic acids, polysaccharides, and lipids.
- Macromolecules
Compounds with large molecular weights, typically greater than 10,000 daltons.
- Micromolecules
Smaller biomolecules with molecular weights less than 1,000 daltons.
- Polysaccharides
Long chains of monosaccharides, serving as energy storage or structural materials.
- Lipids
Fatty acids and their derivatives, primarily involved in energy storage and membrane structure.
- Proteins
Polymers of amino acids that perform a variety of functions in living organisms.
- Nucleic Acids
Polymers made of nucleotide monomers that carry genetic information.
Reference links
Supplementary resources to enhance your learning experience.
