SUMMARY
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Chemical Composition of Living Organisms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to discuss the fundamental differences between the chemical composition of living and non-living organisms. Can anyone tell me what primary elements are found in living organisms?

I think it's mainly carbon, hydrogen, and oxygen.

Exactly! These elements are abundant in living tissues and are essential for biomolecules. Now, comparing living tissues to the Earth's crust, what differences can we notice?

While both have similar elements, living tissues have a higher proportion of carbon and hydrogen!

That's right! Remember, this difference highlights the unique biochemical processes in life. A mnemonic to remember these elements is 'CHO' for Carbon, Hydrogen, and Oxygen. Can anyone recall the most abundant chemical in living organisms?

Water!

Exactly! Water makes up a significant part of cellular mass. Great job understanding this foundational concept!
Types of Biomolecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the elements, let’s discuss small biomolecules. What types of biomolecules do you know?

There are amino acids, sugars, and fatty acids!

Good! These small biomolecules serve as building blocks. Amino acids, for instance, come together to form proteins. How many amino acids are typically found in proteins?

Twenty different amino acids!

Correct! Here is a memory aid: 'APPS' – Amino Acids, Proteins, Polysaccharides, Sugars. These represent the vital types of biomolecules in living organisms. Can any of you name a type of macromolecule?

Polysaccharides!

Exactly! They store energy and form structural components and play crucial roles in plants and fungi.
Roles of Macromolecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive into macromolecules. What are the three main types found in living organisms?

Proteins, nucleic acids, and polysaccharides.

Well done! Let’s talk about proteins first. What roles do they play?

Enzymes and structural proteins!

Right again! Proteins serve various functions, including enzymes, antibodies, and hormones. Now, what about nucleic acids?

They store genetic information!

Exactly! DNA and RNA are essential for passing hereditary information. To remember this, think of 'Genetic Material = DNA & RNA'. Lastly, who can summarize the purpose of polysaccharides?

They are mainly energy storage and provide structural support!

Great job! Polysaccharides like starch and cellulose showcase their dual roles in plants.
The Role of Enzymes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s focus on enzymes. What are enzymes primarily made of?

They're mostly proteins!

Correct! Enzymes catalyze biochemical reactions in cells. How do they enhance the reaction rate?

By lowering the activation energy!

Exactly! A good mnemonic for this is 'EASE' – Enzymes Activate Substrate Energy. Can anyone discuss the specificity of enzymes?

They are specific to their substrates!

Great observation! Enzymes are tailored to catalyze specific reactions, which is vital for metabolic pathways.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the elemental composition of living organisms, noting that while they contain the same elements as non-living matter, the relative abundance of elements like carbon and hydrogen is much higher in living systems. It emphasizes the types of biomolecules and macromolecules that exist within these organisms and their various roles.
Detailed
In-Depth Summary:
Living organisms, despite their vast diversity, have a commonality in their chemical composition and metabolic processes. When analyzing living tissues, researchers have discovered a similarity in elemental composition with non-living matter; however, the higher relative abundance of carbon, hydrogen, and oxygen in living systems differentiates them. Water is noted as the most abundant chemical in these organisms.
The section also counts thousands of small biomolecules which include amino acids, sugars, fatty acids, glycerol, nucleotides, and nitrogen bases. Out of 20 different amino acids, most proteins in living organisms are heteropolymers comprised of these amino acids.
In terms of macromolecules, there are three main classes identified: proteins, nucleic acids, and polysaccharides. Each of these plays critical roles, such as structural support, genetic information storage, and energy storage. Furthermore, enzymes, which are primarily proteins or ribozymes (nucleic acids that act as enzymes), are highlighted for their specific catalytic functions within metabolic reactions. These enzymes lower the activation energy required for reactions, thus significantly accelerating the biochemical processes necessary for life.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Chemical Composition of Living Organisms
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Although there is a bewildering diversity of living organisms, their chemical composition and metabolic reactions appear to be remarkably similar. The elemental composition of living tissues and non-living matter appear also to be similar when analysed qualitatively. However, a closer examination reveals that the relative abundance of carbon, hydrogen and oxygen is higher in living systems when compared to inanimate matter.
Detailed Explanation
This chunk highlights that while living organisms exhibit immense diversity, their fundamental chemical makeup is quite similar. When comparing the elements found in both living and non-living matter, both contain many of the same elements, such as carbon, hydrogen, and oxygen. However, living organisms have higher concentrations of these essential elements, particularly carbon, hydrogen, and oxygen, which are key to biological processes.
Examples & Analogies
Think of the chemical composition of living organisms as a recipe for a cake. While different cakes may look and taste very different (like different species of organisms), most cakes share common ingredients such as flour (carbon), sugar (hydrogen), and eggs (oxygen). The proportion of these ingredients will vary from one recipe to another, but almost all cakes will use them.
Abundance of Water and Biomolecules
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The most abundant chemical in living organisms is water. There are thousands of small molecular weight (<1000 Da) biomolecules. Amino acids, monosaccharide and disaccharide sugars, fatty acids, glycerol, nucleotides, nucleosides and nitrogen bases are some of the organic compounds seen in living organisms. There are 20 types of amino acids and 5 types of nucleotides.
Detailed Explanation
Water is identified as the most abundant substance in living organisms, crucial for maintaining cellular function and facilitating biochemical reactions. In addition to water, living things consist of a vast array of biomolecules, many of which are small and lightweight (less than 1000 Da). The list includes amino acids, which are building blocks of proteins, sugars including monosaccharides and disaccharides that serve as energy sources, and fatty acids that contribute to the formation of lipids and cell membranes. There are 20 different amino acids used to build proteins, and nucleotides, the building blocks of nucleic acids, can be categorized into five different types.
Examples & Analogies
Consider your body as a complex structure, like a skyscraper. Water acts as the foundation that holds everything together, just like a solid base supports a building. The biomolecules are the various materials used to construct the building—bricks (amino acids), glass (sugars), and steel (fatty acids and nucleotides). Each type of material serves a different purpose but all are essential for the building's integrity.
Types of Biomolecules
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
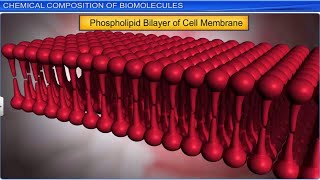
Fats and oils are glycerides in which fatty acids are esterified to glycerol. Phospholipids contain, in addition, a phosphorylated nitrogenous compound.
Detailed Explanation
This part explains types of lipids, specifically fats and oils that are made from glycerol linked to fatty acids through a process called esterification. Phospholipids are a special type of lipid important in forming cell membranes and they contain a glycerol backbone, two fatty acids, and a phosphate group attached to a nitrogenous compound. This unique structure allows phospholipids to form bilayers that serve as cell membranes.
Examples & Analogies
Imagine fats and oils as different types of vehicles. Just like you have cars (fats) designed for long trips and bikes (oils) better for zipping around town, phospholipids are specialized vehicles that manage how cells interact with their environment, ensuring that what comes in and out of the cell is carefully controlled, much like traffic lights controlling vehicle flow.
Macromolecules in Living Systems
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Only three types of macromolecules, i.e., proteins, nucleic acids and polysaccharides are found in living systems. Lipids, because of their association with membranes separate in the macromolecular fraction. Biomacromolecules are polymers.
Detailed Explanation
This section identifies three primary types of macromolecules found in living systems: proteins, nucleic acids, and polysaccharides. While lipids are important for cellular membranes, they are not categorized as traditional macromolecules due to their structural configuration. Macromolecules, such as proteins and nucleic acids, are large and complex molecules made up of smaller units called polymers, which are formed by the repetition of smaller building blocks.
Examples & Analogies
Think of a macromolecule like a long train comprised of many cars that are all connected. Each car represents a smaller subunit (monomer), and together they make a longer train that can carry out significant functions—like proteins that transport materials across a cell or nucleic acids that store genetic information. Just as the train cannot operate effectively with missing or damaged cars, a cell cannot function properly without its full complement of macromolecules.
Hierarchy of Structures and Functions
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Biomacromolecules have a hierarchy of structures – primary, secondary, tertiary and quaternary. Nucleic acids serve as genetic material. Polysaccharides are components of cell wall in plants, fungi and also of the exoskeleton of arthropods. They also are storage forms of energy (e.g., starch and glycogen). Proteins serve a variety of cellular functions.
Detailed Explanation
Here, the summary elaborates on the complexity of biomacromolecules, which feature a structured hierarchy. Proteins exhibit various levels of structural organization—primary (sequential order of amino acids), secondary (pleated sheets and helices), tertiary (3D structure), and quaternary (multiple polypeptide chains). Nucleic acids like DNA store genetic instructions, while polysaccharides such as cellulose provide structural integrity to plants and serve as energy storage molecules in the form of starch and glycogen.
Examples & Analogies
Envision a multi-story skyscraper. Each floor (like the levels of protein structures) serves a purpose, from parking at the ground level (primary structure) to offices and facilities on higher floors (secondary and tertiary structures) culminating in the entire building working together (quaternary structure). Similarly, the genetic blueprint (nucleic acids) provides the foundation for life, while polysaccharides build the structural frame and energy resources for organisms.
Roles of Proteins and Enzymes
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Many of them are enzymes, some are antibodies, some are receptors, some are hormones and some others are structural proteins. Collagen is the most abundant protein in animal world and Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO) is the most abundant protein in the whole of the biosphere.
Detailed Explanation
This section emphasizes the various roles proteins play in living organisms. Enzymes speed up biological reactions, antibodies fight infections, receptors transmit signals, and hormones regulate physiological activities. Collagen is highlighted as the most widespread protein in animals, providing structural support, whereas RuBisCO plays a critical role in photosynthesis, making it the most prevalent protein on Earth.
Examples & Analogies
Think of proteins in a cell as a bustling team of workers in a factory. Each worker (protein) has a specific task—some machines (enzymes) speed up processes, some assemble parts (structural proteins like collagen), and some handle quality control (antibodies). Just as each role is vital for the factory's success, every type of protein is essential for the proper functioning of cells and, ultimately, the whole organism.
Functionality of Enzymes
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enzymes are proteins which catalyse biochemical reactions in the cells. Ribozymes are nucleic acids with catalytic power. Proteinaceous enzymes exhibit substrate specificity, require optimum temperature and pH for maximal activity. They are denatured at high temperatures. Enzymes lower activation energy of reactions and enhance greatly the rate of the reactions.
Detailed Explanation
The summary specifies that enzymes are specialized proteins speeding up chemical reactions within cells, while ribozymes, which are nucleic acids, can also act like enzymes. Enzymes need specific conditions, such as temperature and pH, to function effectively. If the temperature is too high, enzymes can lose their shape (denature), resulting in loss of function. The main attribute of enzymes is their ability to reduce the activation energy required for reactions, significantly increasing the reaction rate.
Examples & Analogies
Consider enzymes as traffic officers directing cars at busy intersections. Just as officers manage and facilitate the flow of traffic safely while reducing accidents (activation energy), enzymes ensure that biochemical reactions happen quickly and efficiently without unnecessary delays. Without them, biochemical processes would be much slower and inefficient.
Genetic Information and Heredity
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Nucleic acids carry hereditary information and are passed on from parental generation to progeny.
Detailed Explanation
In this part, it is highlighted that nucleic acids, such as DNA and RNA, are crucial for carrying genetic information from one generation to the next. They serve as the blueprint for building organisms, determining traits, and guiding cellular functions. When organisms reproduce, they pass on their nucleic acids to their offspring, which provides continuity of life and variation.
Examples & Analogies
Think of nucleic acids as a family recipe book passed down through generations. Each recipe (gene) gives instructions on how to make specific dishes (characteristics) that define the family (organism). Just as the family gathers and shares their methods (DNA transmission), every generation evolves while still sharing core recipes.
Key Concepts
-
Biomolecules: Organic compounds essential for life, including proteins, carbohydrates, lipids, and nucleic acids.
-
Macromolecules: Large biomolecules such as proteins and nucleic acids that play critical roles in organisms.
-
Chemical Composition: The type and abundance of elements in living organisms, showing remarkable similarities despite diversity.
-
Enzymes: Proteins that act as catalysts in biochemical reactions by lowering activation energy.
Examples & Applications
Proteins like collagen serve structural roles in tissues, while enzymes like amylase help in digestion.
Nucleic acids such as DNA store genetic information, and RNA plays a role in protein synthesis.
Polysaccharides like starch serve as energy stores in plants, while cellulose provides structural support.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In living bodies, water flows, with proteins helping life to grow.
Stories
Imagine a tiny town made of different buildings: proteins are the builders, nucleic acids the blueprint, while carbohydrates keep the energy flowing in.
Memory Tools
To remember the types of biomolecules: 'Pencils Need Power - Proteins, Nucleic acids, Polysaccharides'.
Acronyms
Remember CHO for Carbon, Hydrogen, and Oxygen's importance in life.
Flash Cards
Glossary
- Biomolecules
Organic compounds found in living organisms, including amino acids, sugars, and fatty acids.
- Macromolecules
Large biomolecules, primarily proteins, nucleic acids, and polysaccharides.
- Elemental Composition
The types and amounts of elements present in a substance, such as carbon, hydrogen, and oxygen in living matter.
- Enzymes
Proteins that catalyze biochemical reactions by lowering the activation energy.
- Metabolism
The set of life-sustaining chemical reactions in organisms.
Reference links
Supplementary resources to enhance your learning experience.
