How do Enzymes bring about such High Rates of Chemical Conversions?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Enzyme Action
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore how enzymes work. Can anyone tell me what an enzyme is?

Isn't it something that speeds up chemical reactions?

Exactly! Enzymes are biological catalysts that increase the rate of reactions by lowering the activation energy required. Does anyone know what activation energy means?

Is it the energy needed to start a reaction?

Yes! Now, enzymes achieve this by binding substrates at their active sites to form an enzyme-substrate complex.

What happens to the substrate once it binds?

Great question! Once the substrate binds, it often changes shape, which helps in breaking or forming chemical bonds.

So, it goes through a transition state?

Exactly! The substrate transitions through several unstable states before becoming a product. To remember this process, think of ES – 'Enzyme-Substrate' – and then EP, which stands for 'Enzyme-Product'!

In short, enzymes are crucial for facilitating rapid biochemical changes. Remember that they return to their original state after the reaction.
Transition State and Activation Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper into the idea of transition states. Can anyone recall what a transition state is?

Is it the state where the substrate is changing into a product?

Exactly! It's the high-energy state during the transformation. Enzymes help stabilize this state, lowering the needed activation energy. What can you tell me about energy levels during this process?

I think the substrate has to reach a higher energy level to transform into the product, right?

Right on! And when the energy of the product is lower than that of the substrate, it indicates an exothermic reaction. Can anyone give me another example of enzyme action?

Like carbonic anhydrase speeding up the reaction of carbon dioxide and water to form carbonic acid?

Exactly! It increases molecule formation dramatically. Remember, enzymes need to bind the substrate at their active site to catalyze reactions efficiently.
Factors Affecting Enzyme Activity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand how enzymes work, let’s talk about what can affect their activity. What environmental factors do you think might have an impact?

I think temperature and pH might matter!

Correct! Each enzyme has an optimal temperature and pH range. When conditions stray from this range, what happens to the enzyme?

It can become denatured, right?

Exactly! Denaturation permanently alters enzyme structure. Can increasing the substrate concentration also affect enzyme activity?

Yes, but only up to a point. Eventually, the enzyme gets saturated.

Very good! The maximum velocity reached is called V max. Remember, the enzyme activity can also be inhibited by competitive inhibitors, right?

Yes! They compete with the substrate to bind to the enzyme.

Great summary! Environmental conditions are essential in regulating enzyme activities.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains how enzymes catalyze biochemical reactions at high rates. Enzymes work by binding substrates at their active sites, creating an enzyme-substrate (ES) complex that helps transition substrates to products more efficiently than uncatalyzed reactions. Activation energy is reduced, allowing reactions to proceed quickly.
Detailed
Detailed Summary
Enzymes are biological catalysts that significantly increase the rates of chemical reactions in living organisms by lowering the activation energy required for the reaction to occur. Each enzyme possesses a specific active site where substrates bind temporarily, leading to the formation of an enzyme-substrate (ES) complex. This complex is crucial for the transformation of substrate into product. The process of enzyme action can be described in steps:
- Substrate Binding: The substrate binds to the enzyme's active site, inducing a change in the enzyme's shape to accommodate the substrate tightly.
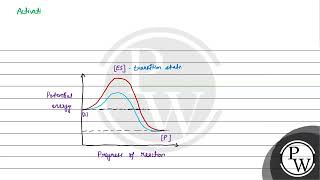
- Transition State Formation: As the enzyme-substrate complex forms, it transitions to a higher energy state known as the transition state, where the substrate undergoes a structural transformation.
- Product Formation: The chemical bonds in the substrate break, and new bonds form, resulting in the production of new chemical products, which are released from the enzyme.
- Enzyme Recovery: After the reaction, the enzyme is free to catalyze another reaction with a new substrate.
This catalytic process allows enzymes to enhance the reaction rate tremendously compared to non-catalyzed reactions, accomplishing the conversion of substrates (S) into products (P) effectively.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
The Role of Enzymes in Chemical Reactions
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To understand this we should study enzymes a little more. We have already understood the idea of an ‘active site’. The chemical or metabolic conversion refers to a reaction. The chemical which is converted into a product is called a ‘substrate’. Hence enzymes, i.e. proteins with three dimensional structures including an ‘active site’, convert a substrate (S) into a product (P). Symbolically, this can be depicted as: S → P.
Detailed Explanation
Enzymes are special proteins that speed up chemical reactions in the body. For every reaction, there is a starting molecule called a substrate. When the enzyme meets the substrate, they interact at a specific region on the enzyme called the active site. This interaction helps convert the substrate into a product, hence facilitating the reaction.
Examples & Analogies
Think of an enzyme like a key and the substrate like a lock. Only the right key (enzyme) can fit into the lock (substrate) to open it (catalyze the reaction). Just as a key turns a lock to open a door, an enzyme changes the substrate into a new product.
Formation of Enzyme-Substrate Complex
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is now understood that the substrate ‘S’ has to bind the enzyme at its ‘active site’ within a given cleft or pocket. The substrate has to diffuse towards the ‘active site’. There is thus, an obligatory formation of an ‘ES’ complex. E stands for enzyme. This complex formation is a transient phenomenon.
Detailed Explanation
For an enzyme to perform its function, the substrate must first collide and bind to the enzyme at the active site, resulting in an enzyme-substrate complex (ES). This is often a temporary state that occurs as the enzyme prepares to convert the substrate into the product. This process is crucial because it positions the substrate in the right way for the chemical reaction to occur.
Examples & Analogies
Imagine a chef (the enzyme) preparing a dish (the product) that requires specific ingredients (the substrate). Before the chef can create the dish, all ingredients must come together on the countertop (the active site) to be chopped and mixed together.
Transition State Formation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
During the state where substrate is bound to the enzyme active site, a new structure of the substrate called transition state structure is formed. Very soon, after the expected bond breaking/making is completed, the product is released from the active site.
Detailed Explanation
Once the substrate is bound to the enzyme, it is altered into a transition state—an unstable state that is a precursor to forming the product. After undergoing the necessary transformations, the enzyme releases the product, returning to its original state to catalyze another reaction.
Examples & Analogies
Think of baking a cake. When you mix the ingredients, you create a batter (transition state) before baking it in the oven. Once baked, you get a cake (the product), which is different from the individual ingredients you started with.
Activation Energy and Enzymes
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Implicit in this statement is the fact that all other intermediate structural states are unstable. Stability is something related to energy status of the molecule or the structure. Enzymes eventually bring down this energy barrier making the transition of ‘S’ to ‘P’ more easy.
Detailed Explanation
Every chemical reaction requires a certain amount of energy to break bonds and initiate the reaction. This energy is known as activation energy. Enzymes lower the activation energy needed, allowing the substrates to convert into products more easily and quickly. By lowering this barrier, enzymes expedite chemical reactions.
Examples & Analogies
Consider a hill that you need to climb to get to the other side. If the hill is steep, it requires more effort (activation energy) to get over it. Now, imagine there’s a path around the hill that gently slopes down, making it easier and faster to reach the other side. This path represents how enzymes reduce the activation energy needed for reactions.
Key Concepts
-
Enzyme Action: Enzymes bind substrates and convert them into products, significantly speeding up reactions by lowering activation energy.
-
Active Site: The specific region on the enzyme shape where substrates fit.
-
Reaction Mechanism: The process involves the formation of the enzyme-substrate complex, followed by conversion to the product.
-
Factors Influencing Activity: Temperature, pH, substrate concentration, and inhibitors can affect enzyme function.
Examples & Applications
Carbonic anhydrase catalyzes the conversion of carbon dioxide and water to carbonic acid rapidly, demonstrating enzyme efficiency.
Enzymes such as amylase help in breaking down starch into sugars in our digestive system.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Enzymes bring energy down low, speeding up reactions, watch them go!
Stories
Imagine an obstacle course – the substrate needs to pass through. Enzymes are like helpful guides, showing the best path and removing barriers.
Memory Tools
A mnemonic to remember the sequence: S → ES → P (Substrate to Enzyme-Substrate complex to Product). 'Some Enzymes Prefer'.
Acronyms
Remember 'ESCP' – Enzyme, Substrate, Complex, Product for stages in enzyme action.
Flash Cards
Glossary
- Enzyme
A biological catalyst that accelerates chemical reactions in living organisms.
- Activation Energy
The minimum energy required to initiate a chemical reaction.
- Substrate
The reactant molecule that an enzyme acts upon.
- Active Site
The specific region of an enzyme where substrate binding occurs.
- EnzymeSubstrate Complex (ES)
The transient structure formed when an enzyme binds to its substrate.
- Transition State
A high-energy state during the conversion from substrate to product.
- V max
The maximum velocity of an enzymatic reaction at saturating substrate concentration.
- Denaturation
The process by which an enzyme loses its functional shape, typically due to heat or pH changes.
- Competitive Inhibitor
A substance that mimics a substrate, competing for the active site of an enzyme.
Reference links
Supplementary resources to enhance your learning experience.
