Factors Affecting Enzyme Activity
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Influence of Temperature on Enzyme Activity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are discussing how temperature affects enzyme activity. Can anyone tell me how enzymes behave at low temperatures?

They become less active, right? They can even become dormant!

Activity increases until it reaches a peak, but if it gets too hot, the enzyme can denature.

Correct! Enzymes have an optimum temperature where they function best. Beyond this, denaturation involves unfolding of the enzyme structure, inhibiting its action. Remember, higher temperatures can be damaging! Let’s remember this with the acronym 'H.O.T = Heat Out = Termination'.

Got it! So, heat causes termination.

Great! Let's move on to how pH affects enzyme activity.
Influence of pH on Enzyme Activity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can someone explain how pH affects enzymes?

Enzymes have an optimal pH range, and if the pH changes too much, it affects their activity.

Exactly! Each enzyme has a specific pH where it works best. Deviations can lead to decreased activity or even denaturation. Anyone remember what enzyme activity looks like graphically in relation to pH?

It looks like a bell curve, right? It shows the peak at the optimum pH.

Precisely! Let’s remember this with 'pH is key, or activity will flee!' Now, let’s discuss substrate concentration next.
Substrate Concentration Effects
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s look at how substrate concentration affects enzyme activity. What happens when we increase substrate concentration?

At first, the rate of the reaction increases.

Correct! The enzyme activity increases until it reaches a maximum rate, also known as Vmax. What happens after that point?

The reaction rate levels off because there aren't enough enzymes to bind with the additional substrate.

Great! Thus, Vmax represents saturation. You can remember this as 'Substrate Up, Activity Stops.'

Got it! So, substrate saturation means no additional activity.
Effect of Inhibitors on Enzymes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about inhibitors. What do we mean by enzyme inhibition?

Inhibitors are substances that reduce enzyme activity.

Exactly! There are different types of inhibitors. What can you tell me about competitive inhibitors?

They look similar to the substrate and compete for the active site!

Correct! Competitive inhibition can decrease the reaction rate significantly. Just remember: ‘When they compete, they take a seat.’ Great job everyone, this wraps our discussion on factors affecting enzyme activity!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explores the key factors affecting enzyme activity, including temperature, pH levels, substrate concentration, and the presence of inhibitors. Each enzyme has optimal conditions under which it operates most efficiently, and deviations from these conditions can decrease its activity.
Detailed
Factors Affecting Enzyme Activity
Enzymes, primarily proteins, catalyze biochemical reactions crucial for life. Several factors can influence enzyme activity by altering the enzyme's structure or its interaction with substrates:
Temperature:
- Enzymes function optimally within specific temperature ranges known as optimum temperatures.
- Activity increases with temperature until it peaks; however, high temperatures can denature enzymes, leading to loss of activity.
pH:
- Each enzyme has an optimum pH at which it is most active. Deviations from this optimal pH can lead to decreased activity or denaturation of the enzyme.
Substrate Concentration:
- As substrate concentration increases, enzyme activity tends to rise until the enzyme becomes saturated; beyond this point, a further increase in substrate does not affect reaction velocity.
Inhibitors:
- Specific chemicals can inhibit enzyme activity. Competitive inhibitors mimic substrates and compete for the enzyme's active site, reducing the overall reaction rate.
Together, these factors illustrate the delicate balance required for optimal enzyme function, which is vital for numerous biochemical reactions pivotal to life.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Effect of Temperature and pH on Enzyme Activity
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
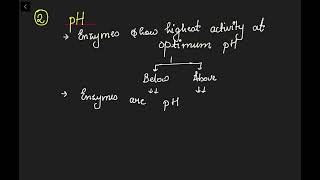
Enzymes generally function in a narrow range of temperature and pH. Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH. Activity declines both below and above the optimum value. Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Detailed Explanation
Enzymes are proteins that catalyze chemical reactions. They are most effective within specific temperature and pH ranges. The optimum temperature is the temperature at which an enzyme performs the best. If the temperature is too low, enzymes may not work efficiently as they become inactive. Conversely, if the temperature is too high, the enzyme structure can become damaged or denatured, rendering it useless. Similarly, pH levels affect enzymes; they have an optimum pH range, and straying too far from this range can decrease their activity.
Examples & Analogies
Think of enzymes like a car engine that runs best at a certain temperature. If it's too cold, the engine may struggle to start, and if it's too hot, it can overheat and stop working. Just like engines have optimal running temperatures, enzymes have theirs, which is crucial for their performance in biological processes.
Substrate Concentration and Enzyme Activity
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
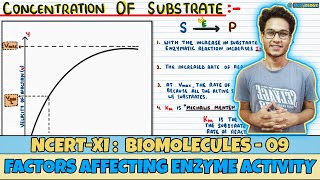
With the increase in substrate concentration, the velocity of the enzymatic reaction rises at first. The reaction ultimately reaches a maximum velocity (Vmax) which is not exceeded by any further rise in concentration of the substrate. This is because the enzyme molecules are fewer than the substrate molecules and after saturation of these molecules, there are no free enzyme molecules to bind with the additional substrate molecules.
Detailed Explanation
When more substrate molecules are available, they can bind to enzymes, increasing the reaction rate. However, after a certain point, all enzymes are occupied, and adding more substrate doesn't speed up the reaction any further; this is known as the maximum velocity (Vmax). The reaction rate levels off because there are no free enzymes left to work on additional substrate. This concept highlights the importance of enzyme availability relative to substrate concentration.
Examples & Analogies
Imagine a busy restaurant where each waiter can serve only one table at a time. As more customers (substrates) come in, the waiters (enzymes) can serve them quickly at first. But eventually, if customers keep arriving and all waiters are busy, no additional customers can be served until a waiter finishes serving a table. Similarly, in enzymatic reactions, once enzymes are busy with substrates, adding more substrates won't help until those enzymes are free.
Influence of Chemical Inhibitors on Enzyme Activity
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The activity of an enzyme is also sensitive to the presence of specific chemicals that bind to the enzyme. When the binding of the chemical shuts off enzyme activity, the process is called inhibition and the chemical is called an inhibitor. When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor.
Detailed Explanation
Enzymes can be inhibited by chemicals that interfere with their action. Inhibitors can either prevent the enzyme from working or reduce its activity. Competitive inhibitors closely mimic the substrate's structure, competing for the active site of the enzyme. This competition prevents the actual substrate from binding, leading to reduced enzymatic activity. Understanding how these inhibitors work is crucial in fields like medicine, where they can be used to control enzyme-related diseases.
Examples & Analogies
Think of a competitive inhibitor as someone trying to take your seat in a theater. If another person (the inhibitor) sits in your designated seat (the active site), you can’t sit down and enjoy the show (the chemical reaction). Thus, the theater (the enzyme) operates less effectively when there are too many people trying to take the same seat, just like enzymes work less efficiently when inhibitors occupy their active sites.
Key Concepts
-
Enzyme Activity: The rate of biochemical reactions catalyzed by enzymes, influenced by various factors.
-
Optimum Conditions: Specific temperature and pH ranges where enzymes function best.
-
Substrate Saturation: The point at which increases in substrate concentration no longer increase the reaction rate.
-
Inhibitors: Substances that decrease enzyme activity, affecting reaction rates.
Examples & Applications
Example of how temperatures above optimum can denature an enzyme leading to loss of function.
Demonstration of how changing pH affects the rate of enzyme reactions through experiments.
Illustrating substrate concentration effect through graphical representation of enzyme kinetics.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In cold, enzymes slow, in heat, they won't show.
Stories
Once, in a lab, enzymes thrived in the warmth, but when temp rose beyond their cozy zone, they lost their magic and couldn't catalyze anymore.
Memory Tools
P.T.S.I. for remembering factors - 'P' for pH, 'T' for Temperature, 'S' for Substrate concentration, and 'I' for inhibitors.
Acronyms
CAT = Conditions Affecting Temperature - used to remember factors affecting enzymes.
Flash Cards
Glossary
- Enzyme
A protein that acts as a catalyst to accelerate a biochemical reaction.
- Denaturation
The process where the structure of a protein is altered, leading to loss of its biological function.
- Optimum Temperature
The temperature at which an enzyme exhibits maximum activity.
- Optimum pH
The pH level at which an enzyme functions most effectively.
- Inhibitor
A substance that decreases or inhibits the activity of an enzyme.
- Competitive Inhibitor
An inhibitor that resembles the enzyme's substrate and competes for the active site.
- Substrate Concentration
The amount of substrate present that can be converted by the enzyme.
- Vmax
The maximum reaction rate of an enzyme in a substrate-saturated condition.
Reference links
Supplementary resources to enhance your learning experience.
