Enzymes
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Enzymes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start with the basics. Enzymes are primarily proteins that serve as catalysts in biochemical reactions. Can anyone tell me what a catalyst does?

A catalyst speeds up a chemical reaction without being consumed.

Exactly! Enzymes lower the activation energy of reactions, which means they help the reactions happen faster. Remember the term 'activation energy' as it’s crucial for understanding enzyme functionality.

So, how do they actually work?

Excellent question! Enzymes have a specific region called the 'active site' where substrates bind. This is crucial for the enzyme's function.

Can you give an example of an enzyme?

Sure! One of the most important enzymes in our body is carbonic anhydrase, which helps convert carbon dioxide and water into carbonic acid. This reaction is vital for controlling pH in blood.

So to recap, enzymes are proteins that catalyze reactions by lowering activation energy, and they do this through their active sites.
Factors Affecting Enzyme Activity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know what enzymes are, let's explore what affects their activity. What do we think happens if we change the temperature or pH?

If the temperature is too high, the enzyme might denature?

Exactly! High temperatures can lead to denaturation, which means losing the enzyme's functional structure. Each enzyme has an optimum temperature and pH for activity.

What’s an optimum pH for an enzyme?

The optimum pH varies from one enzyme to another. For example, pepsin works best in acidic conditions. Always remember enzymes can be very sensitive to changes in their environment.

Can anybody summarize what we’ve learned about enzymes and the factors affecting their activity?

Enzymes have optimal conditions for functioning, and changes can lead to denaturation or reduced activity!

Great summary!
Enzyme Classification and Cofactors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's classify enzymes. They are grouped based on the kind of reactions they catalyze. Can anyone list some classifications?

There are oxidoreductases, transferases, and hydrolases!

Correct! Those are key classes. Each enzyme fits into one of these classes based on its function. What about cofactors? Does anyone know what they are?

Are they the non-protein helpers that some enzymes need?

Exactly! Cofactors can be metal ions or organic molecules that are essential for the enzyme’s activity. Without them, the enzyme might not function.

So, would an enzyme be active without its cofactor?

Not at all! That brings us to the concept of apoenzymes, the protein part of an enzyme without its cofactor. Can someone summarize today’s learning on enzyme classification and cofactors?

Enzymes are categorized based on their reactions, and many need cofactors to activate.

Excellent! Let’s continue building on this knowledge!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses enzymes' structure, function, and significance in biological reactions. Enzymes lower the activation energy required for reactions, making them more efficient than uncatalyzed reactions. The concept of the active site, the impact of various factors on enzyme activity, enzyme classification, and the role of cofactors in enzymatic function are explored.
Detailed
Enzymes
Enzymes are primarily proteins that catalyze biochemical reactions in living organisms, significantly increasing reaction rates. In this section, we delve into the fundamental concepts surrounding enzymes, enhancing our understanding of biological processes.
Structure of Enzymes
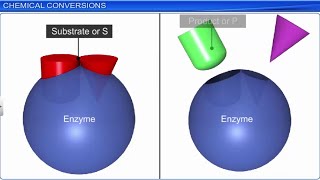
Almost all enzymes are proteins that possess a complex three-dimensional structure, with a specific region known as the active site where substrate molecules bind. The shape and charge of the active site are crucial for the enzyme's functionality.
Function and Mechanism
Enzymes work by lowering the activation energy required for a reaction to occur, which allows reactions to proceed much faster than they would in their absence. The basic schematic of enzyme action can be summarized as:
E + S ⇌ ES ⇌ EP → E + P
Where E represents the enzyme, S is the substrate, ES is the enzyme-substrate complex, EP is the enzyme-product complex, and P is the product of the reaction.
Factors Affecting Enzyme Activity
Enzymes exhibit maximum activity at specific conditions (optimum temperature and pH). Changes in these parameters can lead to denaturation and loss of activity. The relationship between substrate concentration and enzyme activity is characterized by reaching a maximum reaction rate (V_max) when there are no available active sites left for additional substrate.
Types and Classes of Enzymes
Enzymes are classified into several categories based on the reactions they catalyze, such as oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. Each has a specific function and purpose in biochemical pathways.
Role of Cofactors
Many enzymes require non-protein components, called cofactors, for their activity. These cofactors can be metal ions or organic molecules (coenzymes) that assist in the enzyme's catalytic function.
In conclusion, enzymes play a pivotal role in facilitating metabolic reactions essential for life, and understanding their mechanisms provides insight into cellular processes.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Enzymes
Chapter 1 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Almost all enzymes are proteins. There are some nucleic acids that behave like enzymes. These are called ribozymes.
Detailed Explanation
Enzymes are primarily composed of proteins, which are large molecules made up of amino acids. Some unique nucleic acids, referred to as ribozymes, also have enzymatic properties. This means that while the majority of enzymes function as proteins, there are exceptions with nucleic acids playing a similar role.
Examples & Analogies
Think of enzymes like specialized machines in a factory. Most machines are built from durable materials like metal (proteins), but sometimes there are innovative tools made from different materials (nucleic acids) that can also do the job.
Enzyme Structure
Chapter 2 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An enzyme like any protein has a primary structure, i.e., amino acid sequence of the protein. An enzyme like any protein has the secondary and the tertiary structure.
Detailed Explanation
Just like every building has a blueprint (primary structure), enzymes have a sequence of amino acids that determine their specific structure. These sequences then fold into secondary structures (such as helices) and tertiary structures (the overall three-dimensional shape), which are critical for the enzyme's function.
Examples & Analogies
Imagine a piece of origami art. The flat paper (amino acid sequence) gets folded in specific ways (secondary and tertiary structures) to create beautiful shapes. If the folds are wrong, the art may lose its intended look and function.
Active Site of Enzymes
Chapter 3 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When you look at a tertiary structure you will notice that the backbone of the protein chain folds upon itself, the chain criss-crosses itself and hence, many crevices or pockets are made. One such pocket is the ‘active site’.
Detailed Explanation
Enzymes have specific regions known as 'active sites' which are pockets or grooves formed in their folded structure. These active sites are vital because they are where substrates (the molecules upon which enzymes act) fit, allowing for the catalytic action to take place.
Examples & Analogies
Think of the active site like a lock on a door. The right key (substrate) must fit into the lock (active site) for the door to open (reaction to occur). If the key doesn't fit, the door cannot be opened.
Enzyme Catalysis and Reaction Rates
Chapter 4 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enzyme catalysts differ from inorganic catalysts in many ways, but one major difference needs mention. Inorganic catalysts work efficiently at high temperatures and high pressures, while enzymes get damaged at high temperatures (say above 40°C).
Detailed Explanation
Enzymes are highly efficient catalysts that can significantly speed up chemical reactions. Unlike inorganic catalysts that can handle extreme conditions, enzymes are sensitive to temperature and pH levels. Excessive heat can denature enzymes, leading to a loss of function.
Examples & Analogies
Imagine a delicate flower in a garden. It thrives under specific conditions (optimum temperature and pH), but if exposed to extreme heat or cold, it wilts and dies. Similarly, enzymes function well within their preferred conditions, and outside those conditions, they become ineffective.
Chemical Reactions and Enzyme Activity
Chapter 5 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Catalysed reactions proceed at rates vastly higher than that of uncatalysed ones.
Detailed Explanation
Enzymes catalyze reactions much faster than they would occur naturally. For instance, the enzyme carbonic anhydrase can convert carbon dioxide and water into carbonic acid at an astounding rate, demonstrating the power of enzymes in metabolic processes.
Examples & Analogies
Consider a chef expertly chopping vegetables with a high-speed food processor versus using a knife. The food processor (enzyme) speeds up the process noticeably, allowing for quick meal preparation, while chopping by hand (uncatalyzed reaction) takes much longer.
Metabolic Pathways and Enzyme Specificity
Chapter 6 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A multistep chemical reaction, when each of the steps is catalysed by the same enzyme complex or different enzymes, is called a metabolic pathway.
Detailed Explanation
Metabolic pathways consist of a series of interconnected enzymatic reactions that transform a substrate into various products. Specific enzymes speed up each step, facilitating the process. This relationship showcases the specificity of enzymes, where each enzyme is tailored to catalyze a particular reaction.
Examples & Analogies
Think of a treasure hunt involving multiple clues (metabolic steps) leading you to the final treasure (product). Each clue is crucial, and the person guiding you (enzyme) ensures you follow the correct sequence to reach the treasure efficiently.
Factors Affecting Enzyme Activity
Chapter 7 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The activity of an enzyme can be affected by a change in the conditions which can alter the tertiary structure of the protein.
Detailed Explanation
Enzyme activity is influenced by factors such as temperature, pH, and substrate concentration. Changes in these conditions can alter the enzyme's shape and, consequently, its function. Enzymes have an optimal range for these conditions where they operate best.
Examples & Analogies
Visualize how a car engine runs smoothly at certain temperatures and pressures. If conditions deviate too much, the engine may stall or run inefficiently. Enzymes work similarly—ideal conditions lead to smooth 'operation'.
Enzyme Classification and Nomenclature
Chapter 8 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thousands of enzymes have been discovered, isolated and studied. Most of these enzymes have been classified into different groups based on the type of reactions they catalyse.
Detailed Explanation
Enzymes are classified into several categories based on their function, such as oxidoreductases, transferases, hydrolases, and others. This classification helps in understanding their specific roles in metabolic processes.
Examples & Analogies
Think of enzymes as different types of workers in a factory, each specialized for specific tasks. Just as different departments (groups) in a company handle unique jobs, enzymes each play a unique role in biological reactions.
The Role of Co-factors
Chapter 9 of 9
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, there are a number of cases in which non-protein constituents called co-factors are bound to the enzyme to make the enzyme catalytically active.
Detailed Explanation
Some enzymes require non-protein components, or co-factors, to function effectively. These co-factors include metal ions and organic molecules known as coenzymes. They can be essential for the enzyme's activity and help in reaction processes.
Examples & Analogies
Imagine a team where each member has a specific role, and some may require tools (co-factors) to complete their task. Without these tools, even skilled workers may fail to accomplish the job efficiently. Similarly, enzymes often need co-factors to carry out their functions properly.
Key Concepts
-
Enzyme: A protein that catalyzes biochemical reactions.
-
Active Site: The region on an enzyme where substrate molecules bind.
-
Activation Energy: The energy needed to start a chemical reaction.
-
Cofactor: Non-protein molecules that assist enzymes in catalyzing reactions.
-
Apoenzyme: The inactive form of an enzyme without its cofactor.
Examples & Applications
Example of enzyme action: Carbonic anhydrase converts carbon dioxide and water into carbonic acid.
Example of optimal conditions: Pepsin operates best in the acidic environment of the stomach.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Enzymes are proteins, that's quite true, speeding up reactions is what they do!
Stories
Imagine a construction worker (enzyme) who builds houses (catalyzes reactions) in record time. They have a special tool (active site) that fits perfectly with any house plan (substrate), making everything faster and easier.
Memory Tools
Remember 'E.A.S.E' for Enzyme Action: Enzyme - Active site - Speed - Energy (lower activation energy).
Acronyms
ECA
Enzymes Catalyze Actions.
Flash Cards
Glossary
- Enzyme
A biological catalyst that accelerates a chemical reaction.
- Active Site
The specific region on an enzyme where substrates bind and reactions occur.
- Activation Energy
The energy required to initiate a chemical reaction.
- Cofactor
A non-protein substance that helps an enzyme catalyze a reaction.
- Apoenzyme
The protein part of an enzyme, inactive until combined with a cofactor.
Reference links
Supplementary resources to enhance your learning experience.
