Extensive vs Intensive Properties
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Extensive Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we are going to explore extensive properties. Who can tell me what they are?

Are they properties that depend on mass?

That's correct! Extensive properties, like mass, momentum, and energy, vary with the quantity of matter present. Can anyone think of an example?

What about the total amount of energy in a system? It changes when more mass is added.

Exactly! We can summarize this with the acronym 'MEASURE': Mass, Energy, Area, Volume, and is directly related to the amount of substance present. Now, let's proceed to intensive properties.
Diving into Intensive Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, who can explain what intensive properties are?

Intensive properties don't depend on the amount of mass, right?

Correct! For instance, temperature and pressure are independent of mass. Can you think of how we express temperature in a system?

It's the same no matter how much of the substance we have.

Right again! An easy way to remember this is 'TIP': Temperature, Intensity, Properties — which correspond to things not changing with size. Let's summarize the differences before we move on.

Intensive properties remain constant regardless of mass, while extensive properties depend on the quantity of material. This is a vital distinction in fluid mechanics!
Applications to Conservation Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Continuing our discussion, how do intensive and extensive properties relate to conservation laws?

I think extensive properties, like mass, are important for conservation of mass.

That's right! And prestigious equations like the Reynolds transport theorem relate these two types of properties in fluid dynamics. The relationship we often see is that intensive properties can help derive formulas from extensive properties.

So, intensive properties help understand how extensive properties change without being affected by the amount of mass?

Absolutely! This link is crucial when analyzing fluid systems. Let's summarize this with the phrase 'mass measures moments' — emphasizing that understanding these properties helps ensure accurate measurements and assessments.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
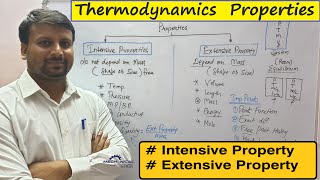
Extensive properties depend on the amount of material present in a system, while intensive properties remain constant regardless of mass. The section explores these concepts through examples related to conservation laws in fluid mechanics.
Detailed
Extensive vs Intensive Properties
This section focuses on the fundamental concepts of extensive and intensive properties in the context of fluid mechanics and energy conservation.
Key Points:
- Definition of Extensive Properties: These properties depend on the mass of a system. They increase or decrease as mass increases or decreases. Examples include mass itself, momentum, and total energy.
- Definition of Intensive Properties: These properties are independent of mass. They remain constant regardless of the quantity of material. Examples include temperature, pressure, and specific energy (energy per unit mass).
- Mathematical Relationships: Intensive properties can often be defined as the ratio of an extensive property to mass. For instance, specific energy (intensive property) is derived from total energy (extensive property).
- Applications in Fluid Mechanics: Understanding these properties is essential for analyzing various conservation laws such as conservation of mass, momentum, and energy. The section establishes the groundwork for more complex topics such as the Reynolds transport theorem.
- Visual Representation: The distinction between these properties is vital when constructing control volumes and solving fluid dynamics problems, which the chapter delves into further with examples and derivations.
Conclusion:
Grasping the difference between extensive and intensive properties is crucial for effective problem-solving in fluid mechanics, particularly when applying conservation laws.
Youtube Videos
![Intensive Properties and Extensive Properties of Fluids [Fluid Mechanics]](https://img.youtube.com/vi/54tra0gDOsM/mqdefault.jpg)







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Defining Extensive Properties
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The extensive property which is considered as proportional to the amount of mass. When you apply extensive properties, that means you are the properties which are proportional to the amount of mass. That means, as mass increases you will have extensive properties going to increase.
Detailed Explanation
Extensive properties are quantities that change when the amount of matter or the mass of a system changes. For example, if you have a larger amount of a substance, properties like mass, volume, and total energy will increase proportionally. Conversely, if you reduce the amount of substance, these properties will decrease accordingly. Thus, extensive properties directly relate to the total mass of a system.
Examples & Analogies
Think about how a bag of flour behaves. If you have a 2 kg bag, it has a certain mass, volume, and energy associated with it. If you increase the bag to 4 kg, all these extensive properties double. In simple terms, extensive properties are like the total weight of groceries in your shopping cart; if you buy more groceries, the weight increases.
Understanding Intensive Properties
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
But when you look at the intensive properties, that means it is independent of mass, that means, which is denoted as . So, if you look it that way, there are two properties, extensive property and intensive property. In intensive property independent to mass or , per unit mass what we are talking about.
Detailed Explanation
Intensive properties are characteristics of a material that do not change regardless of the amount of substance present. These properties remain constant whether you have a small sample or a large one. Examples of intensive properties include density, temperature, and pressure. For instance, if you have a cup of water or a swimming pool full of water, the temperature of the water remains the same irrespective of the quantity.
Examples & Analogies
Imagine tasting sugar in water. Whether you dissolve one spoonful of sugar in a cup or dissolve a whole bag in a large vat of water, the concentration (an intensive property) remains the same until saturation is reached. This illustrates the nature of intensive properties—you can always find the same flavor profile regardless of the size of your sample.
Relationship Between Extensive and Intensive Properties
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We define the difference between extensive property and intensive property. Extensive property we define as B, intensive property we define as b. They have the relationship, simple relation like this, mathematically dB by dm.
Detailed Explanation
The relationship between extensive and intensive properties is fundamental in thermodynamics and fluid mechanics. For instance, if we denote the extensive property as B (like energy) and the intensive property as b (like specific energy), the relationship can be expressed mathematically. The ratio of the extensive property to mass gives the equivalent intensive property. Hence, we can understand that 'b = B/m', meaning specific energy is dependent on total energy divided by mass.
Examples & Analogies
Consider a pizza. The total energy (extensive property) in the pizza is how much energy is in the whole pizza (B). But if you take one slice from that pizza, the energy per slice represents the specific energy (intensive property). As you divide the pizza, the total energy decreases but the energy per slice (intensive property) remains constant if the slices are of equal size.
Key Concepts
-
Extensive Properties: Depend on mass and include mass, momentum, and energy.
-
Intensive Properties: Independent of mass, such as temperature and pressure.
-
Specific Energy: Defined as energy per unit mass, illustrating a key intensive property.
-
Conservation Laws: Fundamental principles that describe the conservation of mass, momentum, and energy.
Examples & Applications
Mass is an extensive property; if you have 10 kg of substance, it has 10 kg of mass, whereas 5 kg has 5 kg.
Temperature remains constant regardless of how much substance you have; e.g., water at 100°C is at the same temperature whether in a cup or a large pot.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Mass and momentum grow, that's how we know extensive flows.
Stories
Imagine a large pot of soup. The temperature stays the same no matter how full it is or how much you serve out. That’s an intensive property!
Memory Tools
Use 'MEASURE' for extensive properties: Mass, Energy, Area, Volume, proportional to amount.
Acronyms
'TIP' reminds us of Intensive properties
Temperature is Independent of mass.
Flash Cards
Glossary
- Extensive Property
A property that depends on the amount of mass in a system, such as mass, momentum, or total energy.
- Intensive Property
A property that is independent of mass, such as temperature, pressure, or specific energy.
- Specific Energy
Energy per unit mass, representing an intensive property related to energy conservation.
- Conservation Laws
Fundamental principles (like conservation of mass, momentum, and energy) that govern the behavior of physical systems.
Reference links
Supplementary resources to enhance your learning experience.
