Crude Oil Distillation Process
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Overview of Crude Oil Distillation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about the crude oil distillation process, which is essential for producing bitumen. Can anyone tell me why distillation is necessary?

Is it to separate different hydrocarbons in crude oil?

Exactly! Distillation helps us separate lighter hydrocarbons from heavier ones. What's the first step in this process?

Is it preheating the crude oil?

Correct! Preheating crude oil to around 400°C is crucial for efficient separation. Remember, we want to make it easier to separate lighter components. You can think of this as heating soup before straining it!
Atmospheric Distillation Explained
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about atmospheric distillation. After preheating, what happens to the crude oil?

It goes into the distillation column, right?

That's right! In the distillation column, lighter hydrocarbons boil off and are collected separately. Can anyone name some of these lighter hydrocarbons?

Gasoline, kerosene, and diesel!

Perfect! Those are all products we get from atmospheric distillation. To help you remember, think of the acronym **GKD** for Gasoline, Kerosene, and Diesel.
Understanding Vacuum Distillation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

After atmospheric distillation, we have a heavy residue left over. What is this called?

Long residue?

Close! It’s known as long residue, and we further distill it under vacuum to avoid thermal cracking. Why do you think that’s important?

So we don't break down the heavy hydrocarbons too much?

Exactly! This process separates lubricating oils while leaving us with a heavy residue called short residue or vacuum residue. This residue is crucial for producing bitumen. Always remember, vacuum helps preserve the quality of these heavier materials.
Link to Bitumen Production
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know how the distillation processes work, how can we summarize the connection to bitumen production?

The vacuum residue is the feedstock for bitumen production.

Correct! It becomes essential in road construction and other applications. Can anyone think of why understanding this process is important for engineers?

It helps them know how to use bitumen properly in construction, right?

That's exactly it! Engineering decisions heavily rely on understanding how bitumen is produced.
Final Thoughts and Review
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To finish our discussion, let's review what we learned about the distillation process. Can anyone recap the steps involved?

First, preheat the crude oil, then do atmospheric distillation, and finally vacuum distillation.

Excellent! And what does the vacuum distillation leave us with?

The vacuum residue!

Right again! Understanding these steps ensures that engineers use bitumen effectively in their projects. Great job today, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the crude oil distillation process used in bitumen production, detailing steps like preheating, atmospheric distillation, and vacuum distillation. Understanding this process is crucial for the efficient production of bitumen for various applications in road construction and other industries.
Detailed
Crude Oil Distillation Process
The crude oil distillation process is the fundamental method for producing bitumen, a crucial material used in road construction and other applications. It comprises several key steps:
- Preheating of Crude Oil: Crude oil is initially heated to approximately 400°C, making it more conducive to separation.
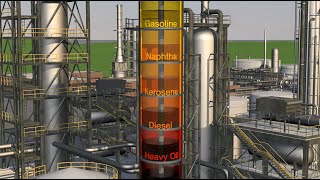
- Atmospheric Distillation: The heated crude oil enters a distillation column where lighter hydrocarbons, such as gasoline, kerosene, and diesel, are separated based on their boiling points.
- Vacuum Distillation: The residue from the atmospheric distillation process, referred to as long residue, undergoes a second distillation under vacuum conditions. This step prevents thermal cracking and effectively separates lubricating oils, leaving a heavy residue known as short or vacuum residue, which is the feedstock for bitumen production.
This process is essential for obtaining bitumen, which is valuable in various fields like civil engineering, specifically in road construction. Understanding the distillation process allows engineers to utilize bitumen effectively.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Preheating of Crude Oil
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Crude oil is first heated to around 400°C.
Detailed Explanation
The first step in the crude oil distillation process involves heating the crude oil to a high temperature of about 400°C. This heating is necessary because it prepares the crude oil for the next phase of the distillation process, where it will be divided into various fractions based on their boiling points. Heating the crude oil increases the energy of the molecules, making them more likely to convert into vapor, which is crucial for separation.
Examples & Analogies
Think of heating crude oil like boiling water for making pasta. Just as you need to heat the water to a certain temperature to start boiling the pasta, crude oil needs to reach high temperatures before it can be distilled into different products.
Atmospheric Distillation
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The heated crude enters a distillation column where lighter hydrocarbons (like gasoline, kerosene, diesel) are separated by their boiling points.
Detailed Explanation
In this phase, the hot crude oil enters a distillation column, a tall tower designed for efficient separation of components based on their boiling points. As the crude oil rises through the column, it cools, allowing lighter hydrocarbons to condense at various levels. Lighter fractions, like gasoline, kerosene, and diesel, have lower boiling points and rise to the top, while heavier fractions stay lower in the column. This method is key in obtaining various fuel types from crude oil.
Examples & Analogies
Imagine a funnel filled with different sizes of marbles. When you pour in a larger marble (representing heavy hydrocarbons), it takes longer to fall through the funnel than a smaller marble (representing lighter hydrocarbons). Just like the marbles separate based on size, hydrocarbons separate based on boiling points in the distillation column.
Vacuum Distillation
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The residue from atmospheric distillation, known as long residue, is further distilled under vacuum to avoid thermal cracking. This separates lubricating oils and leaves behind a heavy residue known as short residue or vacuum residue, which becomes feedstock for bitumen production.
Detailed Explanation
After atmospheric distillation, the remaining substance, called long residue, undergoes vacuum distillation. Operating under reduced pressure decreases the boiling points of the components, allowing for further separation without excessive heat that could cause thermal cracking (breaking down of hydrocarbons). During this process, lighter lubricating oils are recovered, and the leftover heavy fraction, known as short residue or vacuum residue, is what is eventually used to produce bitumen.
Examples & Analogies
Think of boiling a soup to concentrate the flavors. If you boil it too hard, you risk burning or breaking down the ingredients. By reducing the heat and pressure (similar to vacuum), you can concentrate the soup flavors effectively while keeping all the original taste intact. This is how vacuum distillation preserves valuable components in long residue.
Key Concepts
-
Crude Oil Distillation: A fundamental process to separate hydrocarbons in crude oil for various byproducts, including bitumen.
-
Preheating: The first step in distillation, where crude oil is heated to enhance separation efficiency.
-
Atmospheric and Vacuum Distillation: Two key steps in fractionating crude oil into reusable materials.
-
Vacuum Residue: The leftover product from vacuum distillation, crucial for bitumen production.
Examples & Applications
In atmospheric distillation, gasoline vaporizes and separates at a much lower temperature than heavier residue, illustrating how boiling points determine separation.
The use of preheated crude oil means engineers can optimize their refining processes, allowing for better quality bitumen.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To make bitumen that’s top of the line, heat crude oil, watch it intertwine.
Stories
Imagine a chef (representing the engineer) in a kitchen (refinery) heating a pot of ingredients (crude oil). As the soup simmers, they skim off the lighter ingredients and, with special tools, separate the heavier parts to create a perfect sauce (bitumen).
Memory Tools
Remember HAD – Heat, Atmosphere, Distillation. These are the first three critical steps in the distillation process.
Acronyms
Use the acronym **GKD** for Gasoline, Kerosene, Diesel to recall the lighter products obtained from atmospheric distillation.
Flash Cards
Glossary
- Preheating
The initial heating of crude oil to prepare it for distillation, typically to around 400°C.
- Atmospheric Distillation
A distillation process that separates lighter hydrocarbons from crude oil based on their boiling points, performed at atmospheric pressure.
- Vacuum Distillation
A separation process that distills heavy residues under a vacuum to prevent thermal cracking, yielding lubricating oils and heavy residues like vacuum residue.
- Long Residue
The heavy residue remaining after atmospheric distillation, which undergoes further processing.
- Short Residue
The heavy residue left after vacuum distillation, used as feedstock for bitumen production.
Reference links
Supplementary resources to enhance your learning experience.
