Charge is conserved
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Conservation of Charge
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, everyone! Today, we will explore a crucial principle in electrostatics: conservation of charge. Can anyone tell me what this principle states?

I think it means that charge can't be created or destroyed?

Exactly! The conservation of charge means that the total charge in an isolated system remains constant. Whenever charge seems to appear or disappear, it's simply being transferred from one body to another. Let's think of some examples. What happens when you rub a balloon on your hair?

The balloon gets charged, right?

Yes! The balloon gains negative charge while your hair loses some charge. The total charge remains the same. Can anyone recall the term used to describe this process?

It's called electric charge transfer!

Great! Remember, the charge gained by one object equals the charge lost by another. This is the essence of charge conservation.

Let’s summarize: Conservation of charge tells us charge cannot be created or destroyed, only transferred. This principle is essential for understanding how electricity works.
Electric Interactions in Isolated Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In an isolated system with multiple charged bodies, how do you think the interaction between them affects the total charge?

I guess the total charge should stay the same, just like when two objects touch and some charge transfers.

Correct! Even when charges redistribute among objects in the system due to interactions, the overall charge remains constant. This is the key aspect of charge conservation.

So if I have two objects, and one loses charge, it must go to the other?

Exactly! This means if Object A loses 2 coulombs, Object B must gain 2 coulombs. Any other thoughts on how this principle plays a role in physics?

It seems important in understanding circuits and electrical phenomena!

Right! Charge conservation is fundamental in analyzing electrical circuits. Let's conclude this session by reiterating that in any isolated system, the sum of charges is always conserved.
Real-World Applications of Charge Conservation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about how the conservation of charge is applied in real-world physics. Can anyone give an example?

In batteries! They maintain charge balance while they discharge and recharge.

Absolutely! Batteries rely on the principle of charge conservation. How about in nuclear reactions?

They create new particles, but the charge total remains the same!

Exactly! Even as new charged particles form, the overall charge balance is preserved, whether through transformation or decay processes.

This makes sense when you think about how nature balances everything out.

Great insight! Charge balance is not just an electrical principle; it's a universal one. To wrap up, charge conservation is integral both in theoretical physics and practical applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section on charge conservation explains the fundamental law that the total electric charge in an isolated system remains constant. It illustrates that during various interactions among charged bodies, the sum of the charges before and after any process remains identical, highlighting the principle's significance in understanding electrical phenomena.
Detailed
Charge is Conserved
In the study of electric charges, one fundamental concept is the conservation of charge, which states that electric charge cannot be created or destroyed, only transferred between objects. This principle implies that if two objects are electrically charged through friction, such as when one is rubbed against another, the charge gained by one object is exactly equal to the charge lost by the other.
To elaborate further, consider an isolated system consisting of multiple charged bodies. When these bodies interact — perhaps through contact or induction — while charges may redistribute among them, the net charge of the entire system remains constant. This phenomenon was experimentally established and is crucial for understanding electrical interactions.
Another aspect of charge conservation is the transformation of particles; for instance, during certain decay processes in nuclear physics, particles like neutrons can decay into protons and electrons. Although new charged particles are created in this process, the total charge before and after the transformation remains unchanged, illustrating the conservation principle on a particle level.
The implications of this principle extend far beyond simple electrostatics; it serves as a foundational rule in all electrical interactions and leads to various practical applications in physics and engineering. Understanding charge conservation not only aids in solving electrical circuit problems but also in contextualizing larger systems in electromagnetism.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Conservation of Electric Charge
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
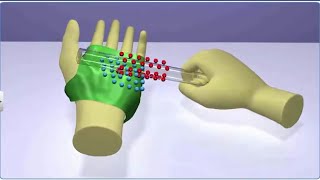
We have already hinted to the fact that when bodies are charged by rubbing, there is transfer of electrons from one body to the other; no new charges are either created or destroyed. A picture of particles of electric charge enables us to understand the idea of conservation of charge. When we rub two bodies, what one body gains in charge the other body loses. Within an isolated system consisting of many charged bodies, due to interactions among the bodies, charges may get redistributed but it is found that the total charge of the isolated system is always conserved.
Detailed Explanation
The principle of the conservation of electric charge states that the total charge in an isolated system remains constant over time. When two objects are rubbed together, like a balloon on your hair, electrons move from one object to another. The amount of charge lost by one object is equal to the amount of charge gained by the other. This means although charges can be redistributed, no new charge emerges or disappears.
Examples & Analogies
Imagine two kids playing with a bag of marbles. If one kid gives two marbles to another, the first kid has two marbles less while the second has two marbles more. The total number of marbles remains unchanged throughout the gameplay, similar to how electric charges are conserved.
Experimental Confirmation of Charge Conservation
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Conservation of charge has been established experimentally. It is not possible to create or destroy net charge carried by any isolated system although the charge carrying particles may be created or destroyed in a process. Sometimes nature creates charged particles: a neutron turns into a proton and an electron. The proton and electron thus created have equal and opposite charges and the total charge is zero before and after the creation.
Detailed Explanation
Through experiments, scientists have shown that while individual charged particles can appear through physical processes, the overall charge in a closed system will not change. For example, when a neutron decays, it transforms into a proton and an electron. The neutron is neutral, the proton is positive, and the electron is negative; their combined charge is zero, showing that charge is conserved even through these transformations.
Examples & Analogies
Think of charge conservation like a balanced scale. If you take weight off one side, you have to add exact weight to the other side to keep it balanced. Similarly, even when particle transformations happen in nature, the total charge remains balanced.
Key Concepts
-
Conservation of Charge: The total charge in an isolated system is constant.
-
Charge Transfer: Charge can only be transferred between objects, not created or destroyed.
-
Total Charge: The sum of the electric charges before and after interactions remains the same.
Examples & Applications
When a glass rod is rubbed with silk, it becomes positively charged as it loses electrons, while the silk gains negative charge.
In nuclear decay, a neutron decays into a proton and an electron, without altering the total charge of the system.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Charge cannot disappear or grow, just change hands as we all know.
Stories
Imagine two friends at a fair. One gives their candy to another. They exchange, yet the total candy stays the same, much like electrical charges!
Memory Tools
C1 - Conservation of charge means, Charge Only Transfers, never creates nor destroys.
Acronyms
CCT - Conservation Chart
Charge Can Transfer.
Flash Cards
Glossary
- Conservation of Charge
The principle stating that the total electric charge in an isolated system remains constant over time.
- Electric Charge
A property of matter that causes it to experience a force when near other electrically charged matter.
- Charge Transfer
The movement of electric charge from one object to another.
Reference links
Supplementary resources to enhance your learning experience.
