Physical significance of dipoles
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Electric Dipoles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore electric dipoles. An electric dipole consists of two equal and opposite charges separated by a distance. Can anyone explain why this setup is significant?

It shows how charges can generate an electric field without net charge, right?

Exactly! The dipole moment, which is the product of the charge and separation distance, helps us understand their behavior in electric fields. Any questions on how this might apply?

Do permanent dipoles have the same concept?

Yes! Permanent dipoles have a fixed dipole moment. Water molecules are a classic example. They exhibit interesting behaviors in electric fields.
Electric Field of a Dipole
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we talk about electric fields, remember that the field created by a dipole decreases faster than that of a single charge. Can someone recall the mathematical expression?

Is it something like E = k * (2p / r^3)?

Close! The field depends inversely on r cubed. This difference is crucial in molecular interactions, especially when looking at polar substances.

What about in the equatorial plane?

Great question! The electric field also behaves differently in the equatorial plane. We'll explore that in detail.
Behavior of Dipoles in External Fields
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss how dipoles behave in external electric fields. What happens when a dipole is placed in such a field?

It aligns with the field direction!

Correct! The torque acting on the dipole attempts to align it in the direction of the electric field. Remember, the torque τ is given by τ = p × E. Why is this relationship important?

It shows how dipoles can contribute to material properties like polarization!

Exactly! Understanding this helps us in fields such as chemistry and material science.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Electric dipoles comprise two equal and opposite charges separated by a distance, playing a vital role in the behavior of molecules in electric fields. This section explores the electric field attributes of dipoles, the concepts of dipole moment, and their applications in polar molecules, highlighting why and how these dipoles generate specific electrical and physical phenomena in different materials.
Detailed
Detailed Summary of Physical Significance of Dipoles
Electric dipoles are defined as pairs of equal and opposite charges (q and -q) separated by a distance (2a). The significance of dipoles emerges from their non-zero dipole moment, which is crucial in understanding several physical and chemical properties of molecules. The direction of the dipole moment vector (p) is conventionally taken to be from the negative charge to the positive charge.
Key Points Covered in This Section:
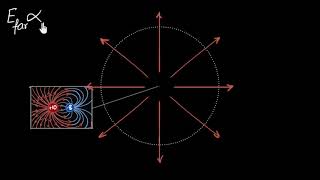
- Electric Field of a Dipole: The electric field produced by a dipole indicates how it interacts with other charges. Notably, the strength of a dipole field falls off faster than that of a point charge, specifically as 1/r³ at large distances.
- Permanent Dipoles: Some molecules, like water (H₂O), possess permanent dipoles due to the non-coinciding centers of positive and negative charges within the molecule, which is significant in defining their interactions in electric fields.
- Behavior in Electric Fields: Dipoles in external electric fields experience a torque that tends to align them with the direction of the field. This is expressed mathematically as torque (τ) being equal to the vector cross-product of the dipole moment (p) and the electric field (E).
- Applications: The physical characteristics of dipoles lead to practical applications, such as their role in the electrification of materials via polarization, impacting their behaviors in static electric fields.
Understanding dipoles assists in grasping broader concepts in electrostatics, including molecular structure, electric interactions, and material properties.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Electric Dipoles
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In most molecules, the centres of positive charges and of negative charges lie at the same place. Therefore, their dipole moment is zero. CO and CH4 are of this type of molecules. However, they develop a dipole moment when an electric field is applied.
Detailed Explanation
An electric dipole is created when there is a separation of charges, with a positive and a negative charge that are not at the same location. In molecules like carbon monoxide (CO) or methane (CH4), the positive and negative charges are balanced, resulting in no net dipole moment. However, when they are subjected to an external electric field, they can become polarized, meaning the charges can shift slightly in response to the field, creating a temporary dipole moment.
Examples & Analogies
Think of a rubber band that is at rest and doesn't stretch (no dipole moment). When you apply a force to stretch it (the electric field), it elongates (develops a dipole moment). This is similar to how some molecules respond to electric fields—they develop a dipole moment when influenced by an external field.
Permanent Electric Dipoles
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In some molecules, the centres of negative charges and of positive charges do not coincide. Therefore they have a permanent electric dipole moment, even in the absence of an electric field. Such molecules are called polar molecules. Water molecules, H2O, is an example of this type.
Detailed Explanation
Certain molecules like water (H2O) have a permanent dipole moment because their structure does not allow the positive and negative charges to align perfectly. This results in a permanent charge separation, creating a dipole. Water, as a polar molecule, has regions of partial positive and negative charge, which leads to its unique properties, such as high surface tension and solvent capabilities.
Examples & Analogies
Consider a magnet: one end is 'north' (positive) and the other is 'south' (negative). Just as a magnet has a permanent nature to its poles, polar molecules like water have a consistent charge separation in their structure, which affects how they interact with other substances.
Applications and Importance of Dipoles
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Various materials give rise to interesting properties and important applications in the presence or absence of electric field.
Detailed Explanation
The interplay of electric dipoles in materials has significant implications in various fields such as chemistry and physics. For instance, dipoles are critical in explaining phenomena like solubility, boiling points, and interactions between different substances. In the presence of an electric field, dipolar materials can align themselves and exhibit properties that can be harnessed in technologies like capacitors and sensors.
Examples & Analogies
Imagine a crowd of people moving through a busy corridor. If a sudden announcement announces a change in the direction of exit, some people (dipoles) will quickly turn and align themselves with the new direction, optimizing the flow. Similarly, in materials, dipoles align under an external electric field, leading to significant changes in their physical properties.
Key Concepts
-
Electric Dipole: A configuration of two equal and opposite charges.
-
Dipole Moment: A measure of the separation of positive and negative charge in a system.
-
Permanent Dipole: A dipole that exists without any external field.
-
Torque of a Dipole: The product of the dipole moment and the electric field.
Examples & Applications
Water as a polar molecule exhibits permanent dipole moments due to uneven distribution of charge.
Electric field strength of a dipole decreases as 1/r³ as you move away from it.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Dancing dipoles align with ease, In electric fields, they move like breeze!
Stories
Imagine a tug-of-war with two friends on opposite sides of a rope (the dipole), pulling towards each other yet balanced, illustrating the concept of an electric dipole.
Memory Tools
DIP - Dipole = Distance x Identity of Positive charge.
Acronyms
P.E.D. - Polarity, Electric field direction, Dipole moment.
Flash Cards
Glossary
- Electric Dipole
A pair of equal and opposite charges separated by a distance.
- Dipole Moment (p)
A vector quantity representing the strength and direction of a dipole.
- Permanent Dipole
A dipole that has a constant dipole moment in the absence of an external electric field.
- Torque (τ)
The rotational force experienced by the dipole in an electric field.
Reference links
Supplementary resources to enhance your learning experience.
