Potential Temperature and Mixing Height
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Atmospheric Stability
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's begin with atmospheric stability. Who can tell me what we mean by stability when discussing air parcels?

Is it how an air parcel behaves as it rises?

Exactly! Atmospheric stability indicates how an air parcel behaves when it ascends. It depends on the temperature gradient in the environment. Can anyone explain what we mean by temperature gradient?

It's the rate at which temperature changes with height, right?

Correct! The temperature gradient is critical in determining stability. If the air gets cooler with height, the air parcel can rise; if not, it will tend to sink. Let’s remember that, using the acronym 'STAB' for Stability, Temperature, Adiabatic Behavior.
Adiabatic Process and Lapse Rate
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's look at adiabatic processes. Who can explain what an adiabatic process means?

I think it means that no heat is exchanged with the surroundings?

Exactly! In an adiabatic process, the air parcel cools as it rises without absorbing heat from the environment. The dry adiabatic lapse rate, or what we note as -0.0098 °C/m, remains consistent. Can anyone suggest why this is significant for us?

It helps us understand how temperature affects pollutant behavior!

That's right! Understanding the lapse rate is crucial for modeling how pollutants disperse in the atmosphere.
Potential Temperature Concept
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss potential temperature. Does anyone know how potential temperature is defined?

Is it the temperature adjusted for a specific pressure?

Precisely! It's the temperature an air parcel would have if moved to a given pressure, typically standard pressure at sea level. Why do you think this is useful?

It helps normalize temperatures so we can analyze and compare different air parcels at varying altitudes!

Exactly! This normalization is key in understanding stability and predicting environmental impacts.
Mixing Height and Its Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s tackle mixing height. What do we mean by mixing height?

Is it the height where environmental lapse and adiabatic lapse rates intersect?

Correct! This height indicates how far pollutants can effectively disperse. Why is this important?

It impacts air quality and helps predict where pollutants will settle or if they will rise further!

Exactly right! Understanding mixing height is crucial for effective environmental monitoring and forecasting.
Plume Dynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s wrap up with plume dynamics. How does mixing height influence plume shapes?

Different mixing heights mean different dispersion shapes?

Correct! The interaction of mixing height with the actual source height affects how pollutants spread. Can anyone summarize why this is significant?

It helps predict the concentration of pollutants at different distances from the source!

Exactly! This is key for anticipating impacts on health and environmental policies.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section delves into the notions of potential temperature and mixing height in relation to atmospheric processes, demonstrating how stability and temperature gradients affect pollutant transfer in the air. It discusses the principles of adiabatic processes and environmental lapse rates, contributing to a foundational understanding of dispersion models in air.
Detailed
Potential Temperature and Mixing Height
This section elaborates on significant atmospheric concepts crucial for understanding pollutant transfer dynamics in the air, including potential temperature and mixing height.
- Atmospheric Stability: Stability refers to the behavior of an air parcel as it ascends. It is primarily influenced by the temperature gradient, encapsulated by the environmental lapse rate.
- Adiabatic Processes: The ideal case of adiabatic cooling describes how an air parcel cools as it rises, without heat exchange with its environment. The dry adiabatic lapse rate is approximately -0.0098°C/m, establishing a constant cooling rate as altitude increases.
- Potential Temperature: Defined as the temperature an air parcel would have if brought to a standard reference pressure (e.g., sea level), it provides a normalized measure to assess stability variations.
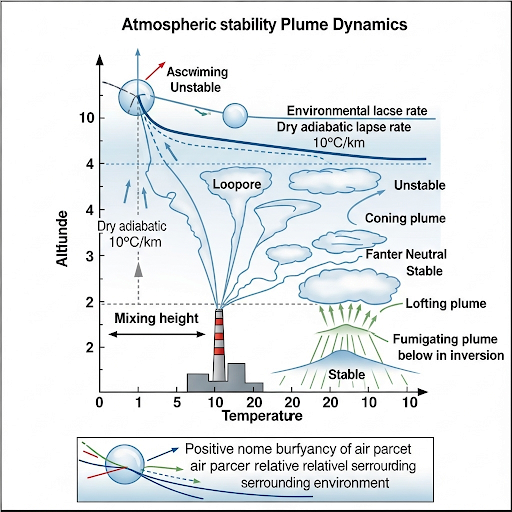
- Mixing Height: This is the elevation where the environmental lapse rate intersects with the adiabatic lapse rate. It indicates the height at which pollutants are effectively dispersed and is vital for modeling plume shapes and behaviors over time.
- Plume Dynamics: The section also highlights how understanding the mixing height and stability helps predict the movement and concentration of pollutants over time and space, grounding the need for effective environmental monitoring.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Potential Temperature
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Potential temperature is defined like this: theta equals T0. This is the temperature corrected to a particular pressure, so the pressure with reference to sea level pressure. So it’s a temperature of an air parcel at temperature T1 and pressure P1, if it is moved to pressure P2. Therefore, it is similar, it is a corrected temperature for pressure.
Detailed Explanation
Potential temperature is a way to understand the temperature of an air parcel when it is moved from one pressure to another. It helps normalize the temperature by compensating for pressure changes, making it easier to compare temperatures at different altitudes. If you have an air parcel at a certain temperature (T1) and pressure (P1), and you want to know what its temperature would be at a different pressure (P2), you use the concept of potential temperature to make that comparison.
Examples & Analogies
Imagine if you had a balloon filled with air at sea level (where the pressure is high) and took it up a mountain (where the pressure is lower). The air inside the balloon expands and cools as it rises. The potential temperature helps us understand what the temperature of the air would be if we could control the pressure changes, allowing us to compare it directly to other parcels of air at different heights.
The Role of Environmental and Adiabatic Lapse Rates
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The mean mixing height is defined as the place where the intersection of the environmental lapse rate and the adiabatic lapse rate happens. This is the plume, and the boundary of this section can be discussed further.
Detailed Explanation
The mean mixing height is critical because it is where the atmospheric conditions (environmental lapse rate) meet the theoretical lapse rate (adiabatic lapse rate) of a rising air parcel. This intersection indicates the height at which the air becomes unstable and begins to mix more vigorously. Essentially, it defines the boundary layer where pollutants may mix and disperse in the atmosphere.
Examples & Analogies
Think of the mixing height like the surface of a stirred pot of soup. When you start stirring, the ingredients mix more at the top and less at the bottom. Just like the soup, the air near the surface is affected more by temperature changes than the air higher up. The mixing height tells us how high the mixing effect goes before the air stabilizes again.
Understanding Plume Shape and Behavior
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We also looked at different shapes from this thing. This is redrawn again to explain some of the things for each of the different types of plume shapes that can occur.
Detailed Explanation
Plume shapes are influenced by various factors, including the environmental lapse rate, the height at which emissions are released, and local meteorological conditions. Understanding these shapes helps predict where pollutants might travel and how they disperse in the atmosphere.
Examples & Analogies
Imagine smoke rising from a campfire. On calm days, the smoke might rise straight up, forming a cylindrical plume. On windy days, the smoke disperses wider and can change shape. Understanding these various plume shapes helps us forecast where the smoke—and any harmful pollutants it carries—might go in the environment.
Mixing Height and Pollution Dispersion
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is the set of conditions for plume shapes. If you know the basic fundamental aspects, you can predict what kind of plume shape you can expect for a given situation.
Detailed Explanation
By identifying key atmospheric conditions and understanding the fundamentals of how air and pollutants behave, we can make educated predictions about how pollutants will disperse. The mixing height plays a crucial role in this process as it dictates where and how pollutants will mix and rise in the atmosphere.
Examples & Analogies
Consider a scenario where a factory is emitting smoke. If the mixing height is low due to temperature inversions, the smoke may not rise and could accumulate in the area, leading to pollution. Conversely, when mixing heights are high, the same pollution might get dispersed over a larger area, reducing its concentration in any specific location. Knowing these dynamics helps in planning and public health responses.
Key Concepts
-
Atmospheric Stability: Indicates air parcel behavior in relation to temperature gradients.
-
Adiabatic Process: Describes how air parcels cool without heat exchange.
-
Dry Adiabatic Lapse Rate: A constant cooling rate of -0.0098 °C/m for rising air.
-
Potential Temperature: Represents a normalized temperature for comparison across different pressures.
-
Mixing Height: A critical height determining effective pollutant dispersion.
Examples & Applications
An example of stable atmospheric conditions would be a clear night, where temperature decreases steadily with height, keeping pollutants close to the surface.
Conversely, an unstable atmosphere can result in rapid dispersion of pollutants, as seen in daytime heating events where surface temperatures rise quickly.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Stable porridge stays, while hot soup will rise, Cooling fast when the altitude flies.
Stories
Imagine a hot air balloon rising; it cools as it goes higher due to no heat transfer, illustrating adiabatic cooling.
Memory Tools
Remember 'STAB' - Stability, Temperature, Adiabatic Behavior to assess atmospheric stability.
Acronyms
P.M.H. for Potential Mixing Height - remember, it helps assess pollutant behavior.
Flash Cards
Glossary
- Atmospheric Stability
The behavior of an air parcel as it ascends, influenced by temperature gradients.
- Adiabatic Process
An thermodynamic process where no heat is transferred to or from the air parcel.
- Dry Adiabatic Lapse Rate
The rate of temperature decrease with altitude for an ascending air parcel, approximately -0.0098°C/m.
- Potential Temperature
The temperature an air parcel would attain if brought to a specified pressure, typically sea level.
- Mixing Height
The height where the environmental lapse rate equals the adiabatic lapse rate, indicating effective pollutant dispersion.
Reference links
Supplementary resources to enhance your learning experience.
