Capillary Effect
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Capillary Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we start with the capillary effect. Who can tell me what happens when a small tube is inserted into water?

The water rises in the tube!

Correct! This rise of water is due to the balance between surface tension and gravity. Can anyone explain why surface tension is important?

Surface tension pulls the liquid up the tube, overcoming gravity.

Exactly. Remember this: 'Surface tension pulls, gravity holds'. We'll use this phrase to remember that dynamic. Now let's explore how this is quantified.
Understanding Pressure Distribution
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about pressure distribution now. Can anyone tell me how pressure varies in a fluid that is at rest?

Pressure is the same at all points on a horizontal surface.

Great! That's key to understanding hydrostatic pressure. The total pressure can be thought of in terms of depth. What do you think happens when we add height?

Pressure increases with depth!

Exactly! The deeper you go, the greater the weight of the fluid above. We can represent this with the formula: Pressure = Height × Density × g. Remember: 'DHD' - Depth Height Density. Let's calculate some examples next.
Calculating Capillary Rise
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s derive how to calculate the height of liquid in a capillary tube. What forces are we balancing here?

We balance the gravitational force with the upward surface tension force.

Correct! The equation involves surface tension, contact angle, tube diameter, and height of rise. We express it as h = (2 * γ * cos(θ)) / (ρ * g * r). What do the variables represent?

h is the height, γ is surface tension, θ is the contact angle, ρ is density, g is gravity, and r is the radius of the tube.

Great job! To remember these components, think 'Happy Cool Granules Return'. Next, let's explore applications of this concept.
Application of Capillary Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s consider how we apply these concepts in real life. Can anyone explain how a mercury barometer works?

It uses mercury to measure atmospheric pressure based on the height of mercury in the tube.

Exactly! The same principles of capillarity help us determine atmospheric pressure. Remember: 'Mercury Measures Mood'. This connects our fluid mechanics concepts to atmospheric science.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section covers the principles governing the capillary effect, emphasizing the balance between gravitational force and surface tension in influencing fluid movement within small tubes. It discusses the calculation of pressure distribution and how atmospheric pressure plays a pivotal role in understanding fluid behavior.
Detailed
Capillary Effect
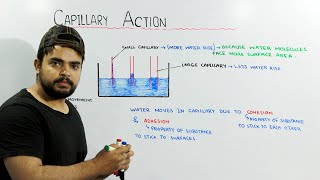
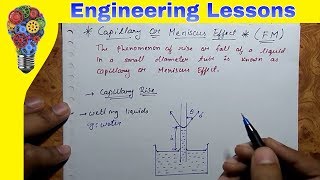
The capillary effect is a phenomenon observed when a liquid rises or falls in a small tube, predominantly due to the interplay between surface tension and gravitational forces. In this section, we explore the conditions under which this effect occurs and its implications in fluid mechanics.
Key Concepts
- Control Volume: Examining a small part of a larger system helps in understanding the pressure distribution effects in fluids at rest.
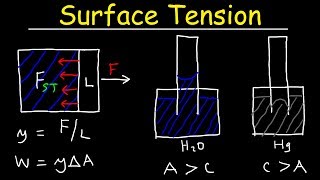
- Surface Tension: The ability of a liquid surface to resist external force, which plays a critical role in capillary action.
- Atmospheric Pressure: It serves as a datum point to measure gauge and vacuum pressures, important for fluid dynamics in real-world applications.
- Hydrostatic Pressure: Variations in pressure due to fluid column heights, leading to effective calculations in capillary tubes.
Pressure Distribution
Pressure variation in fluids can be described using hydrostatic equations, leading to a uniform distribution along horizontal planes. Using these principles, we derive essential equations that describe how pressures behave under different conditions, particularly in scenarios involving capillary tubes and barometers.
In capillary tubes, liquids rise through a height determined by the balance of surface tension forces against gravity. The height can be expressed as a function involving the surface tension, contact angle, and diameter of the tube. Moreover, mercury barometers utilize the same capillary principles to measure atmospheric pressure. This understanding is crucial in fluid dynamics applications, including meteorology and engineering.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Capillary Action
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us consider a control volume and solve the capillary problem. This is what the capillary effect means, germane to how water rises or mercury falls in a small tube. When a small tube is inserted into a fluid, you can see this rise or fall due to two main forces: gravity and surface tension.
Detailed Explanation
Capillary action occurs when a liquid moves upward in a narrow space, such as a thin tube, against the force of gravity. This is primarily due to the balance between the weight of the liquid and the upward force from surface tension. The surface tension pulls the liquid up, while its weight tries to pull it down. The interaction between these forces determines how high the liquid will rise in the tube.
Examples & Analogies
Think of a drink straw. When you place it in a glass of water and sip, the water rises in the straw. This happens because the surface tension of the water, combined with adhesive forces between the water and the straw, pulls the water upward against gravity.
Pressure and Equilibrium in Capillarity
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Inside the tube, if the upper part is exposed to the atmosphere, the pressure there will be equal to atmospheric pressure. At the top of the water column in the tube, the pressure is atmospheric. The weight of the liquid has an upward counterpart from the surface tension force acting in the opposite direction.
Detailed Explanation
In a capillary tube, the liquid pressure at the top remains equal to the atmospheric pressure. If you consider a control volume, you can calculate the upward force from surface tension and equate it to the downward gravitational force acting on the liquid column. Hence, you derive the relationship that defines how high the liquid will rise in terms of both weight and surface tension.
Examples & Analogies
Imagine a tiny sponge submerged in water. As the sponge absorbs water, the forces acting at the surface of the sponge draw water upwards, demonstrating how liquid can move against gravity due to the combined effects of weight and surface tension.
Calculating Capillary Rise
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Equating the upward force from surface tension to the weight of the liquid results in the height of liquid being proportional to the surface tension and inversely proportional to the diameter of the capillary tube. The equation is given by: \( h = \frac{2\sigma \cos(\theta)}{\rho g r} \), where \( h \) is the height, \( \sigma \) is surface tension, \( \theta \) is the contact angle, and \( r \) is the radius of the tube.
Detailed Explanation
The rise of liquid due to capillarity can be calculated using a formula that connects various properties like surface tension, fluid density, gravitational acceleration, and the size of the tube. As the diameter of the tube decreases, the height to which the liquid rises increases, which is counterintuitive but is a direct consequence of how surface tension reacts in narrow spaces.
Examples & Analogies
Think about a small, thin pipe and a wide bucket. If you have two equal amounts of water with one going through the pipe and the other in the bucket, the water in the pipe can rise much higher. The narrower the pipe, the more effectively surface tension can counteract gravity, illustrating how smaller diameters lead to larger heights of liquid due to capillary action.
Mercury Barometer and Atmospheric Pressure Measurement
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a mercury barometer, the same principles of capillarity apply but in a slightly different scenario where mercury is used to measure atmospheric pressure. The mercury does not wet the glass, creating a vacuum at the top of the tube and allowing atmospheric pressure to push the mercury column up to a measurable height.
Detailed Explanation
A mercury barometer uses the capillary effect to measure atmospheric pressure. When the mercury occupies a tube, the height the mercury rises is an indication of atmospheric pressure. The mercury's weight has to be counterbalanced by the atmospheric pressure acting on the liquid surface in the reservoir. Although atmospheric pressure pushes the mercury up, it can only rise to a certain measurable height due to the weight of the mercury acting downwards.
Examples & Analogies
Imagine you have a long, transparent tube half-filled with mercury, with the top sealed to create a vacuum. By observing how high the mercury rises in the tube, you can determine the current atmospheric pressure. This is similar to how a great chef measures ingredients: precision is key, just like accurately measuring air pressure for weather predictions or scientific experiments.
Key Concepts
-
Control Volume: Examining a small part of a larger system helps in understanding the pressure distribution effects in fluids at rest.
-
Surface Tension: The ability of a liquid surface to resist external force, which plays a critical role in capillary action.
-
Atmospheric Pressure: It serves as a datum point to measure gauge and vacuum pressures, important for fluid dynamics in real-world applications.
-
Hydrostatic Pressure: Variations in pressure due to fluid column heights, leading to effective calculations in capillary tubes.
-
Pressure Distribution
-
Pressure variation in fluids can be described using hydrostatic equations, leading to a uniform distribution along horizontal planes. Using these principles, we derive essential equations that describe how pressures behave under different conditions, particularly in scenarios involving capillary tubes and barometers.
-
In capillary tubes, liquids rise through a height determined by the balance of surface tension forces against gravity. The height can be expressed as a function involving the surface tension, contact angle, and diameter of the tube. Moreover, mercury barometers utilize the same capillary principles to measure atmospheric pressure. This understanding is crucial in fluid dynamics applications, including meteorology and engineering.
Examples & Applications
Inserting a thin straw into water demonstrates capillary action as water rises due to surface tension.
Mercury barometers illustrate how atmospheric pressure influences fluid behavior in sealed tubes.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In narrow tubes, water climbs high, surface tension gives it a try.
Stories
Imagine a race where tiny droplets climb a straw. They work together, pulling up against gravity's pull, showcasing the amazing capillary action.
Memory Tools
Remember 'CATS' for Capillary Action, Tension, and Surface.
Acronyms
HATS
Hydrostatic pressure
Atmospheric pressure
Tubular pressure
Surface tension.
Flash Cards
Glossary
- Capillary Action
The ability of a liquid to flow in narrow spaces without external forces.
- Surface Tension
The elastic tendency of a fluid surface which makes it acquire the least surface area possible.
- Hydrostatic Pressure
The pressure exerted by a fluid due to the force of gravity on it.
- Atmospheric Pressure
The pressure exerted by the weight of the air above a given point.
- Gauge Pressure
The pressure relative to atmospheric pressure.
- Vacuum Pressure
Pressure measured below atmospheric pressure.
Reference links
Supplementary resources to enhance your learning experience.
