Forces in Capillary Action
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Fluid Pressure
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to dive into understanding fluid pressure, particularly when the fluid is at rest. Can anyone tell me what happens to the shear stress in such a scenario?

I think the shear stress becomes zero?

Correct! In a fluid at rest, we only consider normal stress acting on surfaces because shear stress contributes nothing. This leads us to focus solely on pressure. Say, if we were to define pressure with the relationship P(x,y,z), what would it tell us?

It tells us how pressure varies throughout the fluid in different directions based on x, y, and z coordinates!

Exactly! This relationship allows us to analyze the pressure field more effectively.
Body Forces vs. Surface Forces
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's distinguish between body forces and surface forces. Can anyone explain what they are?

Body forces act throughout the entire volume of the fluid, like gravity, while surface forces act at the fluid's boundary.

Correct! An example of a body force is gravitational force which acts on the entire fluid. These forces are crucial to understanding how pressure is distributed within a control volume.

So, can we relate this back to gauge and vacuum pressure?

Great connection! Gauge pressure is measured above atmospheric pressure while vacuum pressure is below it, both indicating how these forces affect fluid measurements.
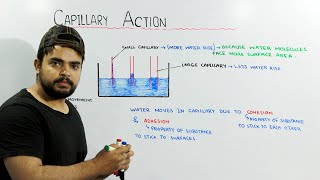
Capillary Action
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's explore capillary action now. Who can describe what happens when we insert a thin tube into water?

The water rises in the tube, right? That's due to surface tension!

Exactly! The balance of gravitational force and surface tension results in the rise of fluid in the tube. What do you think influences how high the fluid can rise?

The diameter of the tube! A smaller diameter will cause a higher rise.

Well stated! This is a key concept in understanding capillary action—the height is inversely proportional to the diameter of the tube. Good job everyone!
Mercury Barometer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss how we measure atmospheric pressure using a mercury barometer. What do you think happens in this device?

I believe it involves measuring the height of mercury in a tube that's inverted?

That's right! The pressure at the top of the mercury column is zero, creating a vacuum. The height of mercury reflects atmospheric pressure based on its weight. What can you derive from the height of mercury?

We can calculate the atmospheric pressure using the height and the weight of mercury!

Correct again! Remember, this practical example bridges theoretical pressure concepts to real-world applications. Excellent work, everyone!
Pressure Distributions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up, how do we find the pressure distribution in a fluid at rest?

By considering only the gravitational force acting downward?

Correct! The pressure varies linearly with depth in a static fluid. How can we mathematically express this relationship?

Using the equation P = ρgz!

Exactly! This equation illustrates how pressure increases with depth due to the weight of the fluid above. Great participation today; all your insights were excellent!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explores the pressure distribution in a fluid at rest, emphasizing the role of gravity as a body force and explaining how pressure is affected by shear stress and normal stress. It particularly highlights the concepts of gauge pressure and vacuum pressure in the context of the fluid's interaction with solid surfaces, illustrating capillary action through practical examples.
Detailed
Forces in Capillary Action
In this section, we explore the behavior of fluids at rest, particularly focusing on the pressure field within a control volume, alongside the effects of shear stress and gravity. Initial discussions outline how shear stress is negligible in a static fluid, allowing us to simplify our analysis to normal stress and pressure acting normal to control surfaces. By delving into Cartesian coordinates to represent the pressure as a function of x, y, and z (P(x,y,z)), we can derive the pressure distribution using Taylor series approximation.
The section also distinguishes between body forces and surface forces, with the focus on the implications of pressure on fluid behavior. Understanding gauge pressure, atmospheric pressure, and vacuum pressure is essential, as these concepts indicate how pressure varies relative to the atmospheric levels. The application of these ideas in practical scenarios, such as through the use of a mercury barometer or capillary tubes, illustrates the forces at play, including gravitational force and forces due to surface tension. Notably, capillary action is observed when a liquid rises in a narrow tube, influenced by the balance between gravitational and surface tension forces.
Lastly, the section concludes with an importance on understanding pressure distributions in static condition fluids, and this forms a significant foundation for exploring more advanced fluid dynamics concepts in subsequent discussions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Capillary Action
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now let me come it to the capillary effect as you could have seen some of the books if any of the class 12th levels. What do we do it we just coming the two force components, the force due to the surface tensions and the gravity force of the capillary part.
Detailed Explanation
Capillary action occurs when a liquid moves up or down in a tube or a small space due to the balance of two forces: surface tension and gravity. Surface tension is caused by the attraction between liquid molecules, which allows the liquid to 'climb' up the walls of a narrow tube against gravity. This phenomenon is commonly demonstrated with water in a thin glass tube.
Examples & Analogies
Think about how when you place a straw in a glass of water, you can see the water rise slightly inside the straw. This is due to capillary action, where the water is climbing up against the gravitational pull because of the adhesive forces between the water molecules and the straw material.
Force Components in Capillarity
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These two force components works. One is the gravity force; another is the surface tension force. These two force components works. Now if I consider this is the control volume. That means, I have considered this part as a control volume.
Detailed Explanation
In capillary action, the balance of the upward force created by surface tension and the downward force due to gravity determines how high a liquid rises in a capillary tube. When we define a control volume, we can analyze these forces more easily. The force due to surface tension acts upward while the weight of the liquid column acts downward, and when these forces are balanced, the liquid reaches a stable height known as capillary height.
Examples & Analogies
Imagine a tall glass of water with a very thin straw. If you were to use a light piece of paper to cover the top of the straw and then pull it out, the water will rise slightly in the straw before falling back down. This demonstrates how surface tension can lift the water column upward, countering the gravitational force until a balance is reached.
Pressure and Surface Distribution
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
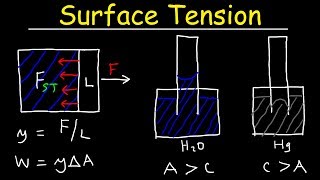
So at the top you have atmospheric pressure. So at the top you have atmospheric pressure, below you have atmospheric pressure. And over this control surface with a radial control surface you will have a pressure distribution will be the hydrostatic pressure distribution starting from zero to the at the z level.
Detailed Explanation
Inside a capillary tube, the pressure at the top and the bottom of the liquid column is equivalent to the atmospheric pressure. As you move down the tube, the pressure increases due to the weight of the liquid column above it. This relationship shows how hydrostatic pressure varies with depth, and it is fundamental for understanding how liquids behave in various scenarios.
Examples & Analogies
Consider a tall glass of syrup and how the pressure at the bottom of the syrup (near the bottom of the glass) is higher than at the surface. This is because the weight of the syrup above pushes down, creating pressure due to gravity, much like how the atmosphere exerts pressure on everything on Earth.
Equating Forces
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So you have the weight of the fluid. Just equate these two part. The upward force due to the surface tension and weight of the fluid. What will get it; weight of the liquid column and equate that to the pressure difference due to surface tension at a height.
Detailed Explanation
To determine how high a liquid will rise due to capillary action, we set the upward force from surface tension equal to the downward force from the weight of the liquid column. This equation allows us to find the relationship between the height the liquid rises, the surface tension of the liquid, and the radius of the capillary tube.
Examples & Analogies
Picture a sponge soaked in water—when the sponge is pressed, the water is forced upwards into the sponge while the gravitational pull tries to keep it down. The amount of water that remains in the sponge demonstrates the balance of these forces at play.
Key Concepts
-
Pressure Field: The pressure inside a fluid that varies with the coordinates of the fluid element.
-
Surface Tension: The force that causes liquids to minimize their surface area and helps in capillary action.
-
Fluid Under Rest: Describes a fluid state where the shear stress is negligible.
Examples & Applications
The mercury barometer measures atmospheric pressure by balancing the weight of mercury in a tube against atmospheric pressure.
Capillary action can be observed when water rises in a thin glass tube due to adhesive forces between the water and glass.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Gauge pressure's the difference, feel the sun's light, above the air, it's quite right!
Stories
Imagine a tiny water droplet climbing up a narrow glass tube, where it's not just the water but the magic of surface tension that helps it reach up high against gravity, showing the interplay between forces!
Memory Tools
Remember G-PV-S! Gauge is Positive View - it’s above the atmospheric where pressure is found.
Acronyms
CAP
for Capillary
for Action
for Pressure - all balances in a small tube!
Flash Cards
Glossary
- Pressure
The force per unit area exerted by a fluid against a surface.
- Gauge Pressure
The pressure measured relative to atmospheric pressure.
- Vacuum Pressure
The pressure that is below atmospheric pressure.
- Capillary Action
The ability of a liquid to flow in narrow spaces without the assistance of external forces.
- Hydrostatic Pressure
The pressure exerted by a fluid at rest due to the weight of the fluid above.
Reference links
Supplementary resources to enhance your learning experience.
