Addition Reaction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Addition Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore addition reactions. These reactions are crucial when we discuss unsaturated hydrocarbons. Can anyone tell me what an unsaturated hydrocarbon is?

I think it's a hydrocarbon that has double or triple bonds.

Exactly! Unsaturated hydrocarbons contain either double or triple bonds between carbon atoms. During addition reactions, these compounds can add hydrogen. Why do you think this is important?

I guess it helps to create saturated hydrocarbons?

Correct! When hydrogen is added, the carbon atoms can form single bonds, resulting in saturated hydrocarbons. This leads to compounds that are generally more stable.

Remember: 'Add hydrogen, you're saturated!' That's a mnemonic to recall the essence of this process. Shall we dive deeper into a specific example?
Hydrogenation of Vegetable Oils
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's look at a real-world application: hydrogenation of vegetable oils. Who can tell me what we mean by hydrogenation?

Isn't that when we add hydrogen to oil to make it healthier?

Exactly! Vegetable oils are typically unsaturated, containing double bonds. By hydrogenating them with a catalyst like nickel, we convert them into more saturated forms, which is often considered healthier. Why do you think this is marketed as such?

Because animal fats are saturated and can be unhealthy in large amounts, right?

That's right! There’s a significant health aspect to this. So, next time you see 'hydrogenated' on a label, you'll know it's about enhancing health by modifying fats.
The Role of Catalysts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about catalysts, essential players in addition reactions. Does anyone know why we use catalysts?

They speed up reactions without getting consumed, right?

Exactly! Catalysts like palladium or nickel make it easier for hydrogen to bond with the unsaturated hydrocarbon. Can someone explain how this might impact a reaction's efficiency?

It reduces the energy requirement for the reaction, which means it can be done faster!

Well said! Less energy means more practical applications, especially in industries where time and resources matter.
Conclusion and Key Takeaways
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up today’s discussion, addition reactions mainly involve unsaturated hydrocarbons gaining hydrogen to form saturated hydrocarbons, often using catalysts. Can anyone summarize why addition reactions are significant?

They help produce healthier fats from oils and are efficient due to catalysts!

Exactly! Remember, understanding these reactions helps us appreciate the compounds we consume daily. 'Hydrogenation equals health!' What a crucial takeaway!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores addition reactions where unsaturated hydrocarbons add hydrogen, facilitated by catalysts like palladium or nickel. It also discusses the application of this reaction in the hydrogenation of vegetable oils to produce healthy cooking oils.
Detailed
Addition Reaction
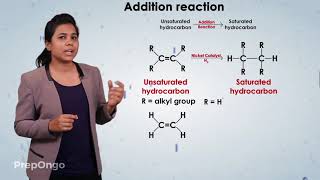
In the realm of organic chemistry, addition reactions play a critical role, particularly involving unsaturated hydrocarbons. These reactions occur when unsaturated hydrocarbons, which possess one or more double or triple covalent bonds, react with hydrogen in the presence of catalysts such as palladium or nickel. The primary result of this reaction is the formation of saturated hydrocarbons, where all carbon atoms in the molecule are bonded with single covalent bonds.
A commonly referenced example of addition reactions is the hydrogenation of vegetable oils, which usually consist of long unsaturated carbon chains. This process is essential for converting these oils into saturated fats, which are often labeled as 'healthy' oils compared to the saturated fatty acids prevalent in animal fats. This is not only significant for culinary purposes but has implications for health and nutrition as well.
Understanding the addition reactions elucidates the versatility of carbon compounds and how they interact within various biological and physiological pathways, affecting our health through dietary choices.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Hydrogen Addition to Unsaturated Hydrocarbons
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons.
Detailed Explanation
Unsaturated hydrocarbons are compounds that contain one or more double or triple bonds between carbon atoms. When these compounds react with hydrogen, they can convert those double or triple bonds into single bonds. This process is called hydrogenation and typically requires a catalyst, which is a substance that increases the rate of the reaction without being consumed. Common catalysts for this reaction include palladium or nickel. As a result of this reaction, the unsaturated hydrocarbons become saturated hydrocarbons, which are generally more stable.
Examples & Analogies
Think of unsaturated hydrocarbons like a sponge that can soak up more water. When you add hydrogen to an unsaturated hydrocarbon in the presence of a catalyst, it’s like filling that sponge until it's completely full of water - now it can't absorb any more, similar to how a saturated hydrocarbon can't react further under normal conditions.
Industrial Application in Hydrogenation
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst.
Detailed Explanation
In the food industry, hydrogenation is often used to convert liquid vegetable oils into solid or semi-solid fats, such as margarine or shortening. This is done by carefully adding hydrogen gas to the liquid oil while using a nickel catalyst. This process not only changes the physical state of the oil but also affects its shelf life and stability, making it suitable for various cooking and baking applications. The conversion helps oils resist rancidity, improving their longevity on grocery shelves.
Examples & Analogies
Imagine you have a juicy, tender piece of meat. If you leave it out too long, it can spoil and become unsafe to eat. Hydrogenation is like putting a sealed cover over meat, preserving it for longer! By transforming oils through hydrogenation, the shelf life is extended, similar to how vacuum sealing keeps food fresh.
Health Implications of Fat Types
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains.
Detailed Explanation
Vegetable oils, which are high in unsaturated fats, can help lower bad cholesterol levels and are considered healthier by many nutritionists. In contrast, animal fats are primarily saturated fats, which can raise bad cholesterol levels and lead to heart disease if consumed in excess. Understanding the types of fats we consume helps us make healthier dietary choices. Choosing oils that maintain their unsaturated state is recommended for overall heart health.
Examples & Analogies
Consider vegetable oils as fruits you pick fresh - they’re vibrant and healthy. In contrast, animal fats are like overly ripe fruit that has started to spoil - they may seem fine at first but can lead to health problems if chosen too often. By incorporating healthy oils, we’re choosing the 'fresh fruit' option for our diets!
Key Concepts
-
Addition Reaction: A key type of reaction in organic chemistry where unsaturated hydrocarbons become saturated through hydrogen addition.
-
Hydrogenation: The process of adding hydrogen to unsaturated hydrocarbons, significant for food processing.
-
Catalysts: Substances that increase the rate of a chemical reaction without undergoing permanent changes.
Examples & Applications
Hydrogenation of vegetable oils to create healthier cooking oils.
Catalysts like nickel used in the hydrogenation process.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hydrogen's the key, add it with glee, unsaturated to saturated, it's plain to see!
Stories
Once upon a time, in the land of Fats, unsaturated oils were sad and flat. They wanted to be friends with hydrogen, but they needed a catalyst to help them begin. With the help of Nickel, they bloomed, turning into saturated fats, now healthy and groomed!
Memory Tools
CATS: Catalysts Add To Saturation – a way to remember the role of catalysts in addition reactions.
Acronyms
H2S
Hydrogen to Saturation – easily recall that hydrogen is crucial for the transition to saturated hydrocarbons.
Flash Cards
Glossary
- Addition Reaction
A reaction where unsaturated hydrocarbons add hydrogen to become saturated hydrocarbons.
- Unsaturated Hydrocarbon
A type of hydrocarbon that contains double or triple carbon-carbon bonds.
- Hydrogenation
The process of adding hydrogen to unsaturated compounds, often using a catalyst.
- Catalyst
A substance that increases the rate of a chemical reaction without being consumed.
Reference links
Supplementary resources to enhance your learning experience.
