Some Important Carbon Compounds – Ethanol and Ethanoic Acid
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ethanol
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're learning about ethanol, commonly known as alcohol. Can anyone tell me where we might encounter ethanol in our daily lives?

We use it in beverages, right? Like beer and wine!

And it's in some medicines too!

Exactly! Ethanol is a versatile compound. It’s a colorless liquid and boils at 351 K. Let's not forget to mention that ethanol is also a good solvent! What's one effect of drinking too much alcohol?

It can make you dizzy or even pass out.

Right! It's important to consume it responsibly. Let's talk about its chemical properties now.
Reactions of Ethanol
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

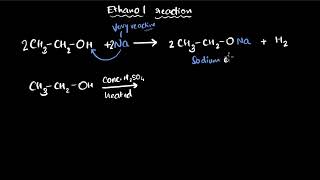
Ethanol can react with sodium to produce sodium ethoxide. When sodium reacts with ethanol, what gas gets released?

Hydrogen gas!

Good job! Let's also look at a dehydration reaction. When we heat ethanol with sulfuric acid, what product do we get?

Ethene!

That's right! Ethene is a building block for many plastics. Remember, ethanol needs to be handled carefully due to potential central nervous system effects.
Introduction to Ethanoic Acid
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

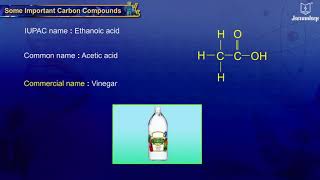
Now, let’s shift our focus to ethanoic acid, also known as acetic acid. Do any of you know what household item contains ethanoic acid?

Vinegar!

I love using vinegar in salads!

Absolutely! Ethanoic acid is the main component in vinegar, typically found in a 5-8% solution. Ethanoic acid also has a freezing point of 290 K, so it can freeze in cold conditions.
Reactions of Ethanoic Acid
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive into the reactions involving ethanoic acid. One fascinating reaction is esterification. Can anyone explain how this occurs?

When it reacts with an alcohol like ethanol, it forms an ester?

Exactly! And what about its reaction with bases?

It produces a salt and water!

Correct! And ethanoic acid also releases carbon dioxide when it reacts with carbonates. Why do you think that’s useful?

It’s a way to test for the presence of an acid!

Yes! Great observations, everyone!
Comparison of Ethanol and Ethanoic Acid
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's compare ethanol and ethanoic acid. While both are organic compounds, how do their acidic properties differ?

Ethanol is not acidic, but ethanoic acid is a weak acid.

Ethanol has health effects at high doses, while ethanoic acid is safe in food.

Very true! Understanding these compounds gives us insight into not just their usage in chemistry but also in everyday life.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Ethanol, commonly known as alcohol, plays a significant role in beverages and pharmaceuticals. Ethanoic acid, or acetic acid, is recognized for its culinary uses, particularly in vinegar. This section covers their physical properties, reactions, and significance in various contexts.
Detailed
Detailed Summary of Section 4.4
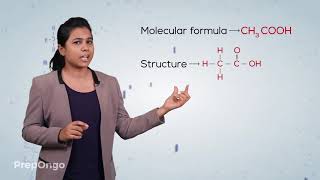
In this section, we focus on two significant carbon compounds: ethanol (C₂H₅OH) and ethanoic acid (C₂H₄O₂).
Ethanol
Ethanol, popularly known as alcohol, is a clear liquid at room temperature with essential applications in beverages, medical solutions, and as a solvent. It has a boiling point of 351 K and is fully miscible with water. Despite its wide use, ethanol can be dangerous in excessive amounts, with pure ethanol potentially being lethal.
- Reactions of Ethanol:
- Reaction with Sodium: Ethanol reacts with sodium to produce sodium ethoxide and hydrogen gas.
- Dehydration Reaction: Upon heating with concentrated sulfuric acid, ethanol dehydrates to form ethene (C₂H₄).
- Impact on Living Organisms: Ethanol consumption can depress the central nervous system, impairing coordination and judgment, while methanol (another alcohol) poses serious health risks.
Ethanoic Acid (Acetic Acid)
Ethanoic acid is a weak organic acid known for its acidic properties and culinary uses, predominantly in vinegar (a 5-8% solution). It has a melting point of 290 K, and in colder environments, it may solidify into its 'glacial' form.
- Reactions of Ethanoic Acid:
- Esterification: Ethanoic acid can undergo a reaction with ethanol in the presence of a catalyst to form an ester.
- Reaction with Bases: It reacts with a base like sodium hydroxide to produce sodium acetate and water, demonstrating its acidic nature.
- Reactions with Carbonates: Ethanoic acid reacts with carbonates and bicarbonates to produce carbon dioxide, which can be tested when the gas passes through lime-water.
Both compounds play crucial roles not only in everyday life but also in industrial applications, enhancing their importance in the study of organic chemistry.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Properties of Ethanol
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ethanol is a liquid at room temperature (refer to Table 4.1 for the melting and boiling points of ethanol). Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks. In addition, because it is a good solvent, it is also used in medicines such as tincture iodine, cough syrups, and many tonics. Ethanol is also soluble in water in all proportions. Consumption of small quantities of dilute ethanol causes drunkenness. Even though this practice is condemned, it is a socially widespread practice. However, intake of even a small quantity of pure ethanol (called absolute alcohol) can be lethal. Also, long-term consumption of alcohol leads to many health problems.
Detailed Explanation
Ethanol, commonly referred to as alcohol, is a significant compound in our lives. It exists as a liquid at room temperature and is present in every alcoholic beverage. Not only does it provide a recreational effect when consumed in small amounts, causing feelings of relaxation or 'drunkenness,' but it also serves as a solvent in many medications, highlighting its usefulness in both social and medical contexts. However, it’s crucial to understand that pure ethanol is dangerous and can be fatal if ingested even in small quantities. Additionally, frequent and excessive consumption leads to several negative health impacts, such as addiction and organ damage, emphasizing the importance of moderation.
Examples & Analogies
Think of ethanol like a fun party guest that everyone likes at first. They help make gatherings enjoyable (like the social acceptance of drinking), but if you let them overstay their welcome or give them too much attention (like drinking too much), they might cause trouble (leading to health issues). It's important to enjoy without letting things go too far.
Reactions of Ethanol
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
(i) Reaction with sodium – 2Na + 2CH3CH2OH → 2CH3CH2O–Na+ + H2
Detailed Explanation
When ethanol reacts with sodium, it produces sodium ethoxide and releases hydrogen gas. This reaction is significant because it showcases how alcohols like ethanol can react with metals, much like water does, only more vigorously. The hydrogen gas released in this process can be detected by its ability to produce a pop sound when a lit splint is brought near it.
Examples & Analogies
Imagine mixing baking soda and vinegar and watching it bubble over! When sodium meets ethanol, it's like having a party where the sodium is excited and creates bubbles of hydrogen gas. Just like how the bubbles can pop when they escape, the hydrogen gas does something similar.
Dehydration of Ethanol
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene – CH3−CH2OH (Heat) → CH2=CH2 + H2O.
Detailed Explanation
This process of dehydration involves removing water from ethanol, resulting in the formation of ethene, a simple alkene. Heated concentrated sulfuric acid acts as a catalyst, speeding up this reaction without being consumed by it. This transformation from an alcohol to an alkene is an essential reaction in organic chemistry, showing how versatile carbon compounds can be.
Examples & Analogies
Consider pouring water out of a sponge to get it dry; here, the ethanol is like the sponge saturated with water, and the sulfuric acid acts as a power tool that helps to extract the water quickly, leaving behind ethene, similar to the dry sponge.
Properties of Ethanoic Acid
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ethanoic acid is commonly called acetic acid and belongs to a group of acids called carboxylic acids. 5-8% solution of acetic acid in water is called vinegar and is used widely as a preservative in pickles. The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter in cold climates. This gave rise to its name glacial acetic acid.
Detailed Explanation
Ethanoic acid, known for its sharp taste and smell, is a key ingredient in food preservation, particularly in vinegar. As a carboxylic acid, it exhibits the properties typical of acids, although it is classified as a weak acid because it only partially ionizes in solution. Its unique freezing point means that in colder conditions, it can solidify, appearing like ice, thus earning it the 'glacial' designation.
Examples & Analogies
Think of glacial acetic acid as a frozen lake in winter—just as the lake can freeze solid but is fluid in warmer weather, ethanoic acid can exist in both solid and liquid states, indicating its versatility. People often recognize vinegar from their kitchen, and its utility parallels our need for preserving food.
Reactions of Ethanoic Acid
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
(i) Esterification reaction: Esters are most commonly formed by reaction of an acid and an alcohol. Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester.
Detailed Explanation
The reaction of ethanoic acid with ethanol in the presence of an acid catalyst produces an ester, which is a compound known for its pleasant fragrances, commonly found in perfumes and flavorings. This reaction exemplifies the esterification process, where water is also produced as a byproduct. The process highlights how these simple carbon compounds can combine to create more complex and aromatic substances.
Examples & Analogies
Consider baking a cake—when you mix different ingredients like flour (ethanoic acid) and sugar (ethanol) in specific proportions, you create something delightful (ester). Just as the cake has a wonderful smell, esters add fragrances to everyday products!
Key Concepts
-
Ethanol: A versatile compound used in beverages and as a solvent, known for its intoxicating effects.
-
Ethanoic Acid: Renowned for its use in vinegar; exhibits weak acidic properties.
-
Reactions: Ethanol reacts with sodium, an acid, producing hydrogen gas, while ethanoic acid undergoes esterification and neutralization.
Examples & Applications
Ethanol is used in hand sanitizers for its antibacterial properties.
Ethanoic acid in vinegar is crucial for food preservation and flavoring.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ethanol in drinks can make you cheer, but too much may make you shed a tear.
Stories
Once there was a curious chef who learned that vinegar (ethanoic acid) added zest to his dishes, making meals a delightful feast.
Memory Tools
To remember the reactions: 'Ethanol Reacts, Ethanoic Interacts!'
Acronyms
EAS
Ethanol
Acidity
Solubility - key features!
Flash Cards
Glossary
- Ethanol
A colorless, volatile liquid also known as alcohol, used in beverages and as a solvent.
- Ethanoic Acid
An organic acid commonly called acetic acid, primarily known for its use in vinegar.
- Esterification
A chemical reaction that produces an ester from an alcohol and an acid.
- Dehydration
A chemical process that removes water from a compound, often used to convert alcohols to alkenes.
Reference links
Supplementary resources to enhance your learning experience.
