Properties of Ethanol
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ethanol
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing ethanol, also known as alcohol. Does anyone know what state of matter ethanol is at room temperature?

It's a liquid, isn't it?

That's correct! Ethanol is a clear liquid with a melting point of 156 K and a boiling point of 351 K. Can anyone tell me where we find ethanol being used?

It's in alcoholic drinks!

And in some medicines because it's a good solvent.

Exactly! It’s used in tinctures and cough syrups as well. Now, what happens if someone drinks too much pure ethanol?

It can be really dangerous, even lethal!

Yes, alcohol poisoning can occur. That's an important point to remember when discussing the social implications of ethanol.

Remember: Ethanol is soluble in all proportions in water—how could we relate this fact?

It likely means it mixes well with many substances used in food and medicine.

Great connection! Understanding the mixture properties will be essential for our next topics.
Chemical Reactions Involving Ethanol
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

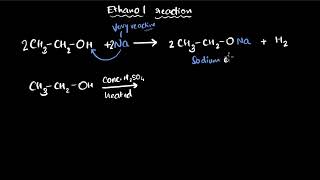
Next, let's explore how ethanol reacts chemically. Student_2, what happens when sodium interacts with ethanol?

It forms sodium ethoxide and hydrogen gas, right?

Correct! Let's write the reaction: 2 Na + 2 C2H5OH → 2 C2H5O-Na + H2. This is a significant reaction. What does the hydrogen gas evolve tell us?

It shows that hydrogen is released, which could also indicate a safety hazard.

Exactly! Safety is paramount. Now, if we heat ethanol with concentrated sulfuric acid, what product do we get?

We get ethene from the dehydration of ethanol!

Spot on! That reaction illustrates how ethanol can act as a precursor to other important compounds. Remember this reaction is also crucial in the octane rating of fuels.
Health Implications of Ethanol
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s focus on the implications of ethanol consumption. Who can tell me about the effects it has on the human body?

It can impair judgment and coordination!

And consuming a lot can lead to addiction and health problems.

That’s absolutely correct! Chronic consumption can lead to liver disease, cardiovascular problems, and more. It’s vital to understand both the positive and negative impacts. What about the lethal effects of pure ethanol?

Even a small amount can be fatal if it's pure ethanol.

Exactly! Hence, the importance of moderation and awareness regarding its consumption. Let’s summarize what we’ve learned about ethanol today.

Ethanol is a versatile compound with significant benefits and serious risks. Its chemical properties and reactions showcase its usefulness while also highlighting necessary caution.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Ethanol is a colorless liquid that serves as a key ingredient in alcoholic beverages and medical formulations. Its interactions with sodium and its reactions under specific conditions, like dehydration, are crucial to understanding its properties and uses. While it is socially popular, its potential health risks and lethal properties in concentrated forms underscore the need for responsible consumption.
Detailed
Ethanol is widely recognized for its roles as an alcoholic beverage and a versatile solvent in pharmaceuticals and other applications. It has a low melting (156 K) and boiling point (351 K), placing it firmly in a liquid state at room temperature. Importantly, ethanol exhibits considerable solubility in water, thereby enhancing its utility. The chemical behavior of ethanol includes the formation of various compounds through specific reactions, such as its interaction with sodium to produce sodium ethoxide and hydrogen gas. Additionally, the dehydration of ethanol using concentrated sulfuric acid leads to ethene production, illustrating the modifications that ethanol can undergo. Despite its social acceptance in moderate amounts as a recreational drug, excessive or pure ethanol can pose severe health risks, including lethality. Thus, understanding both the beneficial and adverse effects of ethanol is critical for its responsible use.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Ethanol
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ethanol is a liquid at room temperature. Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks. In addition, because it is a good solvent, it is also used in medicines such as tincture iodine, cough syrups, and many tonics.
Detailed Explanation
Ethanol is a clear, colorless liquid that is widely recognized for its role in alcoholic beverages. It's important to note that ethanol is not just used for drinking; it serves as an excellent solvent in medicine and various products. This ability to dissolve a wide range of substances makes it valuable in pharmaceuticals, where it helps to deliver active ingredients effectively.
Examples & Analogies
Think of ethanol like a key ingredient in cooking. Just as salt enhances the flavor of food, ethanol enhances the effectiveness of medicines by dissolving them and making their application easier.
Solubility in Water
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ethanol is also soluble in water in all proportions.
Detailed Explanation
Ethanol's solubility in water means it can mix well with water in any ratio. This property arises because ethanol has both hydrophilic (water-attracting) and hydrophobic (water-repelling) parts in its molecular structure. The -OH (hydroxyl) group in ethanol can form hydrogen bonds with water molecules, allowing for complete mixing.
Examples & Analogies
Imagine how sugar dissolves in tea. Just like sugar sweetens tea, ethanol dissolving allows it to be used in beverages and medicines without forming layers; it fully integrates, enhancing the overall effectiveness.
Effects of Consumption
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consumption of small quantities of dilute ethanol causes drunkenness. Even though this practice is condemned, it is a socially widespread practice. However, intake of even a small quantity of pure ethanol (called absolute alcohol) can be lethal. Also, long-term consumption of alcohol leads to many health problems.
Detailed Explanation
Ethanol affects the central nervous system, which is why drinking can lead to intoxication. While moderate consumption may be socially acceptable, excessive intake can lead to severe health issues such as liver damage, addiction, and even death from acute toxicity. Understanding the balance between harm and enjoyment is crucial when dealing with substances like ethanol.
Examples & Analogies
Think about using a cleaning product. Just like strong cleaners can damage surfaces if overused, ethanol can also be harmful when consumed excessively. It's essential to use it responsibly.
Reaction with Sodium
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ethanol reacts with sodium leading to the evolution of hydrogen. With ethanol, the other product is sodium ethoxide.
Detailed Explanation
This reaction demonstrates ethanol's reactivity. When sodium comes into contact with ethanol, it replaces a hydrogen atom in ethanol, forming sodium ethoxide and releasing hydrogen gas. This is an important reaction in organic chemistry as it showcases how alcohols can behave like acids, participating in reactions that generate gases and new compounds.
Examples & Analogies
Consider how baking soda releases carbon dioxide when added to vinegar. Similarly, when sodium meets ethanol, it releases hydrogen, illustrating a transformation that can be useful in various applications, such as creating new compounds in a lab.
Dehydration Reaction
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene.
Detailed Explanation
Dehydration is a chemical reaction where water is removed from a substance. In this case, when ethanol is heated with concentrated sulphuric acid, it loses a water molecule and transforms into ethene, a gaseous alkene. This process is significant in organic synthesis, as it allows chemists to convert alcohols into more complex hydrocarbons.
Examples & Analogies
Imagine making a fruit smoothie. If you add too much water, the flavor can dilute. Taking out water, like in our dehydration example, concentrates the flavor, resulting in a richer, fuller taste. This concept applies to chemistry, too: removing water can create something new and often more useful.
Health Impacts of Ethanol
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When large quantities of ethanol are consumed, it tends to slow metabolic processes and to depress the central nervous system.
Detailed Explanation
Large doses of ethanol can diminish brain function, resulting in lack of coordination, confusion, and drowsiness. Long-term use poses serious risks, including addiction and serious health issues. Ethanol can even harm the liver and lead to a condition known as fatty liver disease.
Examples & Analogies
Think about driving a car; if you're tired, you may not react quickly. In the same way, consuming too much ethanol can slow down your body's responses and decision-making processes, which can lead to accidents or serious health problems.
Industrial Use and Denatured Alcohol
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ethanol is an important industrial solvent. To prevent the misuse of ethanol produced for industrial use, it is made unfit for drinking by adding poisonous substances like methanol to it.
Detailed Explanation
In industrial settings, ethanol is highly valued for its ability to dissolve substances. However, to discourage recreational drinking of industrial alcohol, manufacturers add methanol and dyes to create what’s known as denatured alcohol. This makes the product toxic and unpalatable, thus preventing misuse.
Examples & Analogies
Think of how cleaning products are often bright-colored. Just as we color cleaners to prevent them from being mistaken for beverages, industries add toxic substances to ethanol to keep it safe from misuse.
Ethanol as a Fuel
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Some countries now use alcohol as an additive in petrol since it is a cleaner fuel which gives rise to only carbon dioxide and water on burning in sufficient air (oxygen).
Detailed Explanation
Ethanol burns cleanly, producing primarily carbon dioxide and water when oxygen is present. This characteristic makes it an attractive alternative fuel compared to fossil fuels, which can produce harmful emissions. Using ethanol helps reduce pollution and greenhouse gas emissions.
Examples & Analogies
Consider how electric cars reduce air pollution. Just like they are a cleaner alternative to gasoline cars, ethanol serves as a cleaner alternative to fossil fuels, contributing to a more sustainable energy future.
Key Concepts
-
Ethanol is a liquid at room temperature, useful as a solvent and in alcoholic beverages.
-
Reactions of ethanol with metals and dehydration lead to other compounds with specific applications.
-
Responsible consumption of ethanol is critical due to its potential health hazards.
Examples & Applications
Ethanol is used in hand sanitizers as a disinfecting agent.
In the beverage industry, ethanol is the main component of beer, wine, and spirits.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ethanol, oh, alcohol sweet, in drinks and syrups it can’t be beat.
Stories
A bartender named Ed mixed a drink with ethanol, but warned his customers about drinking too much, highlighting the importance of moderation.
Memory Tools
Remember PHS (Product Hazard Safety) when discussing ethanol: it can be productive as a solvent but hazardous if misused.
Acronyms
E-WEDS
Ethanol is a Water-soluble Ethanol and Drug solvent.
Flash Cards
Glossary
- Ethanol
A clear, volatile liquid commonly known as alcohol, used as a solvent and in beverages.
- Sodium Ethoxide
A chemical compound formed by the reaction of sodium with ethanol, releasing hydrogen.
- Dehydration
The removal of water from a substance, a process ethanol undergoes to form ethene.
- Carcinogenic
A substance capable of causing cancer.
- Solvent
A substance that dissolves a solute, forming a solution.
Reference links
Supplementary resources to enhance your learning experience.
