Homologous Series
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Homologous Series
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore the concept of homologous series. Can someone tell me what they understand by this term?

I think it’s a series of compounds that have similar characteristics?

Correct! A homologous series consists of compounds that share a common functional group, which gives them similar chemical properties. What do you think happens as we add more carbon atoms?

Maybe their physical properties change?

Exactly! For instance, the boiling points increase as we move up the series. Can anyone think of an example of a homologous series?

The alcohols like methanol and ethanol!

Great example! Each of these alcohols differs by a –CH₂– unit. So, remember, in a homologous series, compounds can be systematically compared by their structural differences and properties.
Application of Homologous Series
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss how the concept of homologous series applies in real-life situations. Why do you think understanding this series is important for chemists?

Chemists can predict the properties of new compounds based on their position in the series!

Absolutely! This prediction extends to various properties like reactivity, boiling points, and melting points. Can you think of any implications in industry?

Like creating fuels or plastics that have specific qualities?

Yes, correct! By understanding the homologous series, chemists can design compounds with tailored properties for specific applications.
Characteristics of Homologous Series
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore the characteristics of a homologous series. What are the key features we should be aware of?

They have similar chemical properties and exhibit a gradual change in physical properties, right?

Yes! And it’s also important to note that these changes in physical properties, such as boiling and melting points, can be predicted. Can someone tell me how the first member of a series compares to the subsequent ones?

The first one is usually a gas or liquid and the later members are larger molecules which can be liquids or solids?

Exactly! This trend can be seen in the alkane series as well. This shows how the structure influences physical states based on molecular size.
Homologous Series and Functional Groups
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into how functional groups influence the characteristics of compounds in a homologous series. Who can explain what a functional group is?

It’s a part of the molecule responsible for its chemical reactions!

Exactly! In a homologous series, this group defines the compound's reactivity and properties. Can you think of examples?

Like –OH in alcohols or –COOH in carboxylic acids?

Perfect! Each functional group leads to different behaviors within the same series. The compounds may differ in structure but share a common reactivity pattern.
Concluding Discussion on Homologous Series
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we conclude, why do you think the homologous series is a vital concept in organic chemistry?

It gives us a systematic way to understand and predict how compounds behave!

Exactly! It allows chemists to navigate the vast landscape of compounds with more clarity. By understanding a few members, one can infer the properties of many others!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
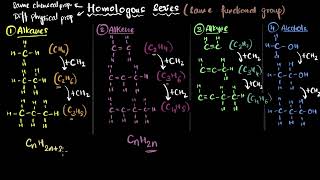
Homologous series refer to a group of organic compounds sharing the same functional group and similar chemical properties, differing by a repetitive structural unit like –CH₂–. The physical properties vary systematically, while chemical properties remain largely unchanged across the series.
Detailed
Homologous Series
The concept of homologous series is crucial in organic chemistry, where it describes a set of compounds that have the same functional group and show a gradual change in their physical properties while retaining similar chemical characteristics. For example, the series of alcohols such as methanol (CH₃OH), ethanol (C₂H₅OH), and so on, provides a clear picture of how compounds differ only by the number of carbon atoms in their chain, each differing by a unit of –CH₂–. As we progress in the series, we see variations in physical properties like boiling and melting points that correlate with molecular weight, but the chemical behavior remains consistent across the series. This systematic nature of the homologous series aids in understanding the structure-function relationship in organic compounds.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Homologous Series
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
You have seen that carbon atoms can be linked together to form chains of varying lengths. These chains can be branched also. In addition, hydrogen atom or other atoms on these carbon chains can be replaced by any of the functional groups that we saw above. The presence of a functional group such as alcohol decides the properties of the carbon compound, regardless of the length of the carbon chain.
Detailed Explanation
A homologous series is a group of compounds that share a specific structural feature, in this case, the same functional group. This means that as the length of the carbon chain increases, the properties of the compounds remain similar due to the presence of the functional group. For instance, compounds like CH₃OH (methanol), C₂H₅OH (ethanol), and C₃H₇OH (propanol) all contain the alcohol functional group (-OH), giving them similar chemical properties.
Examples & Analogies
Think of a family of siblings who have similar traits—they all might have the same eye color or smile. Just like how these siblings share family traits, compounds in a homologous series share a functional group, making them behave similarly in chemical reactions.
Examples of Homologous Series
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For example, the chemical properties of CH₃OH, C₂H₅OH, C₃H₇OH, and C₄H₉OH are all very similar. Hence, such a series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
Detailed Explanation
The compounds methanol, ethanol, propanol, and butanol, all belong to the alcohol homologous series. This means that as we increase the number of carbon atoms from 1 to 4, the core functional group (-OH) remains the same, but the properties like boiling points, melting points, and molecular masses change. This predictability in property trends is one of the most valuable features of homologous series.
Examples & Analogies
Imagine a series of cars of the same model but different sizes: a compact car, a mid-size, and a larger SUV. While each varies in size and weight, they all have the same design and features characteristic of that model, similar to how every alcohol in the series has the same functional group.
Characteristics of Homologous Series
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If we look at the formulae of successive compounds, say – CH₄ and C₂H₆ — these differ by a –CH₂- unit. Similarly, take the homologous series for alkenes. The first member of the series is ethene which we have already come across in Section 4.2.1. What is the formula for ethene? The succeeding members have the formula C₃H₆, C₄H₈ and C₅H₁₀. Do these also differ by a –CH₂- unit?
Detailed Explanation
Each time we move from one compound in a homologous series to the next, there is a consistent increase in the number of carbon and hydrogen atoms by a specific amount—in this case, a –CH₂- unit. This consistent pattern allows chemists to predict the molecular formula for new compounds in the series based on existing compounds.
Examples & Analogies
Think of a staircase where each step up is equivalent to increasing the number of atomic units by the same amount, such as going from one stair to the next. Just as every stair is consistently rising by the same height, every compound in the homologous series consistently adds a specific molecular unit.
Gradation in Physical Properties
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As the molecular mass increases in any homologous series, a gradation in physical properties is seen. This is because the melting and boiling points increase with increasing molecular mass. Other physical properties such as solubility in a particular solvent also show a similar gradation.
Detailed Explanation
As we move up in a homologous series and the molecules gain more carbon and hydrogen, their size increases. This increase in size generally leads to higher melting and boiling points due to stronger intermolecular forces at play. For example, comparing methane (CH₄) and hexane (C₆H₁₄), hexane has a higher boiling point due to the larger number of atoms and the resulting increased van der Waals forces.
Examples & Analogies
Consider the concept of temperature. Just like how larger pots of water take longer to boil than smaller ones because of the increased heat capacity, similarly, larger molecules in a homologous series require more heat to break their bonds and change states.
Chemical Properties Consistency
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
But the chemical properties, which are determined solely by the functional group, remain similar in a homologous series.
Detailed Explanation
Despite the changes in physical properties such as melting points or boiling points, the underlying chemical properties driven by the functional group do not change. This means that reactions such as combustion or oxidation behave similarly across the members of a homologous series.
Examples & Analogies
Think of a sports team where players get promoted to different roles but retain their core skills. Just like how their primary abilities remain the same regardless of their position on the team, the base chemical reactions of a functional group stay constant across the homologous series.
Key Concepts
-
Homologous Series: A group of compounds differing by a -CH₂- unit with similar chemical properties due to a common functional group.
-
Functional Group: The part of a compound that determines its chemical reactivity and properties.
-
Saturated vs Unsaturated: Saturated compounds contain only single bonds, while unsaturated compounds have double or triple bonds.
Examples & Applications
Methanol (CH₃OH), Ethanol (C₂H₅OH), and Propanol (C₃H₇OH) are examples of alcohols in a homologous series.
Alkenes such as Ethene (C₂H₄) and Propene (C₃H₆) exhibit differences in their properties due to the presence of a double bond.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In compounds with similar flips, same group gives them great tips! - a rhyme to remember homologous series.
Stories
Imagine a family of cars, all the same model but different colors; this family shows how similar features allow for differentiation - just like a homologous series of compounds.
Memory Tools
Use 'SAME' for Similar properties' And Molecular changes with Elements.
Acronyms
HOLD - Homologous Series Of Linked Differences.
Flash Cards
Glossary
- Homologous Series
A series of organic compounds that have the same functional group and differ from each other by a constant unit, usually –CH₂–.
- Functional Group
A specific group of atoms within a molecule that is responsible for the characteristic reactions of that molecule.
- Saturated Compounds
Compounds that contain only single bonds between carbon atoms.
- Unsaturated Compounds
Compounds that contain one or more double or triple bonds between carbon atoms.
Reference links
Supplementary resources to enhance your learning experience.
