Soaps and Detergents
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Soaps
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will learn about soaps. Can anyone tell me what soaps are made of?

Are they made of fats or oils?

Exactly! Soaps are often derived from the saponification of fats and oils, transforming them into sodium or potassium salts of fatty acids.

Why do they clean effectively?

Good question! Soaps have a hydrophilic head and a hydrophobic tail, which allows them to interact with both water and oil, effectively cleaning surfaces.

What happens when soap is mixed with water?

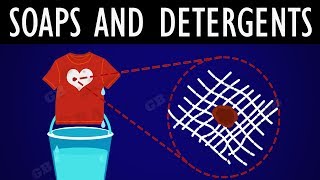
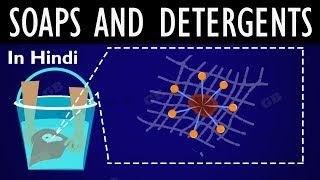
When mixed with water, they form micelles, which are structures that trap oil and grease. Let's remember this as 'Soap is a micelle engineer!'

So the soap wraps around dirt?

Exactly! In summary, soaps work by encapsulating dirt in micelles, making it easy to rinse away with water.
How Soaps Work
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s delve deeper into how soaps work. Can anyone describe micelles?

Are they like tiny bubbles around the dirt?

That's a great way to visualize it! Micelles form when the hydrophobic tails of soap molecules surround oil or dirt while the heads remain in the water.

What does that do?

This structure allows the oily substances to be rinsed away easily. Remember, 'Heads in water, tails in dirt' as a mnemonic for micelles!

Can we use any type of soap in hard water?

Not really. Soaps can react with minerals found in hard water forming scum, which is less effective. Let's keep in mind: 'Soaps falter in hard waters.'

What about detergents?

Detergents are designed to work in hard water and do not form scum. They also create more foam in hard water compared to soap.
Soaps vs. Detergents
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, what’s the difference between soaps and detergents?

Soaps get ineffective in hard water, but detergents don't?

Correct! Detergents work effectively by not forming precipitates. Let's remember: 'Detergents dodge scum!'

So, is detergent always better?

Typically, yes, especially in laundry! But soaps are often more environmentally friendly.

What are detergents made from?

Detergents are typically made from synthetic compounds like sodium sulfonates. They have a similar structure to soaps but are more versatile in hard water.

Can we use detergents for all cleaning?

Certainly! Just remember their ability to perform well even in challenging conditions, like hard water.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Soaps and detergents function as cleansing agents due to their unique molecular structure which allows them to interact with both water and oils. Through the formation of micelles, they effectively encapsulate dirt and oil, enabling their removal. Additionally, the differences between soaps and detergents in terms of their behavior in hard water are discussed.
Detailed
Soaps and Detergents
In this section, we explore the chemical properties of soaps and detergents and their role in cleaning processes. Soaps, made from sodium or potassium salts of long-chain fatty acids, possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) properties. This dual nature allows them to interact effectively with both water and oils, thus facilitating the cleaning of greasy or oily stains.
Micelles Formation
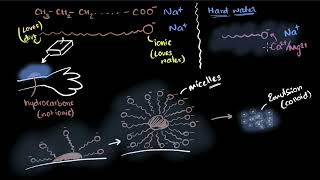
When soap is mixed with water, it forms structures called micelles. In these micelles, the hydrophilic heads of the soap molecules orient towards the water, while the hydrophobic tails point inward, encasing any oil or grease. This configuration allows for the easy washing away of dirt as the soap molecules encapsulate the dirt.
Comparison with Detergents
Detergents serve a similar purpose but are designed to remain effective in hard water conditions, unlike soaps which can form insoluble precipitates with calcium and magnesium ions found in hard water. Detergents, being sodium salts of sulfonic acids or ammonium salts with halogens, prevent the formation of scum and thus create more foam compared to soaps in hard water.
Understanding these principles is vital as they explain the chemical basis behind our everyday cleaning processes.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
The Action of Soap in Cleaning
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Take about 10 mL of water each in two test tubes. Add a drop of oil (cooking oil) to both the test tubes and label them as A and B. To test tube B, add a few drops of soap solution. Now shake both the test tubes vigorously for the same period of time. Can you see the oil and water layers separately in both test tubes immediately after you stop shaking them? Leave the test tubes undisturbed for some time and observe. Does the oil layer separate out?
Detailed Explanation
This chunk describes an experiment demonstrating how soap works for cleaning oily substances. In this exercise, two test tubes each contain water and a drop of cooking oil. One test tube receives a few drops of soap. When both test tubes are shaken, the soap molecules interact with the oil. Soap molecules have two ends: a hydrophilic (water-attracting) end and a hydrophobic (water-repelling) end. The hydrophilic end faces outward into the water while the hydrophobic end attaches to the oil, allowing for the dispersion of the oil in the water and thus forming an emulsion. If left undisturbed, the mixture with soap stays combined, while without soap, oil will separate from water.
Examples & Analogies
Think of washing your hands with soap after cooking. When you have oil or grease on your hands, water alone won’t remove it. But when you use soap, it binds with the grease and allows it to rinse away with water, just like how the experiment shows soap allows oil to mix with water.
Formation of Micelles
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This activity demonstrates the effect of soap in cleaning. Most dirt is oily in nature and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic-end of soap interacts with water while the carbon chain interacts with oil. The soap molecules, thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic-end faces outside. This forms an emulsion in water.
Detailed Explanation
In this explanation, we define micelles, the structures formed by soap molecules in water. The soap molecule’s structure allows it to interact with both oil (dirt) and water. Each soap molecule arranges itself so that its hydrophobic tail is drawn toward the oil droplet while its hydrophilic head remains in water. As more soap molecules gather, they form a spherical structure called a micelle, where the oil is trapped in the center and is surrounded by the soap heads that face outward into the water. This helps remove oily dirt from surfaces, such as clothes or skin.
Examples & Analogies
Imagine washing a plate with greasy residue. Your sponge is coated with soap, which encapsulates the grease in micelles as you scrub. When rinsed under water, these micelles wash away the greasy dirt along with other residues, leaving your plate clean.
Soaps and Hard Water
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Take about 10 mL of distilled water (or rain water) and 10 mL of hard water (from a tubewell or hand-pump) in separate test tubes. Add a couple of drops of soap solution to both. Shake the test tubes vigorously for an equal period of time and observe the amount of foam formed. In which test tube do you get more foam? In which test tube do you observe a white curdy precipitate?
Detailed Explanation
This chunk explores the behavior of soaps in hard versus soft water. When soap is added to distilled (soft) water, it mixes well and produces a good amount of foam. However, in hard water, which contains minerals like calcium and magnesium, the soap reacts with these minerals, forming insoluble salts that precipitate out as a curdy solid instead of foaming, hence reducing its effectiveness. This difference highlights why soaps are less effective in hard water compared to soft water.
Examples & Analogies
Consider how difficult it is to lather soap when showering in a place where the water has high mineral content (hard water) versus using soft water. At home, you might notice that soap doesn't create much lather or feels slimy if you're using hard water, while it works great with soft water, making your bathing experience more pleasant.
Detergents as a Solution
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions, etc. Both have long hydrocarbon chains. The charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain effective in hard water. Detergents are usually used to make shampoos and products for cleaning clothes.
Detailed Explanation
This chunk describes the chemical nature of detergents, which are specifically designed to work in hard water. Unlike soap, detergents do not react with calcium and magnesium ions in hard water to form precipitates. This means that they can work effectively, producing foam and cleaning power even when these minerals are present. Detergents also have a similar two-part structure as soaps, allowing them to interact with both oil and water, thereby helping to remove dirt and grease effectively.
Examples & Analogies
Think about using dishwashing detergent compared to regular soap when cleaning dishes. The detergent cuts through grease effectively, even when the water is hard, while soap may leave residues or not work at all. This is one reason why detergents are preferred in laundry products and dish soaps.
Key Concepts
-
Micelles: Soap molecules arrange to trap dirt and oils when introduced to water.
-
Hydrophobic vs. Hydrophilic: The two distinct parts of soap molecules that interact differently with water and oils.
-
Cleaning Mechanism: Soaps clean by forming micelles that encapsulate dirt and oils for rinsing.
-
Detergents: Synthetic cleaning agents effective in hard and soft water.
Examples & Applications
When soap is added to greasy dishes, micelles form and encapsulate the grease, allowing it to wash off with water.
Detergents can produce foam even in hard water, unlike soaps which may form curdy scum.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Soap helps to cope, with dirt in a swope, micelles in motion, like a ocean!
Stories
Imagine soap as a superhero, with hydrophilic powers to attract water and hydrophobic cape to repel dirt, saving your clothes from greasy villains!
Acronyms
Soaps = 'S.O.A.P.' - Sodium, Oils, Attract people.
Flash Cards
Glossary
- Soaps
Sodium or potassium salts of long-chain fatty acids used for cleaning.
- Micelles
Structures formed by soap molecules that have both hydrophilic and hydrophobic parts, encapsulating dirt and oil for cleaning.
- Detergents
Synthetic cleaning agents that do not form precipitates with hard water ions, ensuring effective cleaning.
- Hydrophilic
Water-attracting part of a soap molecule.
- Hydrophobic
Water-repelling part of a soap molecule.
Reference links
Supplementary resources to enhance your learning experience.
